Intensity Therapeutics, Inc. Announces Publication of Clinical Results of INT230-6 for the Treatment of Metastatic or Refractory Cancers in eBioMedicine, a Lancet Discovery Science Journal
The paper features a comprehensive evaluation of data, including disease control rate, overall survival, immune activation, abscopal effects, tumor necrosis, dose ranging, and safety
The manuscript is open access
The Company will host a webinar with the paper's lead and senior authors from the University of Southern California to discuss the results on Friday, October 31, 2025, at 9:00 AM (see below)
SHELTON, Conn., Oct. 30, 2025 /PRNewswire/ -- Intensity Therapeutics, Inc. (Nasdaq: INTS) ("Intensity" or "the Company"), a late-stage clinical biotechnology company focused on the discovery and development of proprietary cancer therapies using its non-covalent, drug-conjugation technology that creates drug products designed to kill tumors and increase immune system recognition of cancers, announces that eBioMedicine, a Lancet Discovery Science journal, has published the Company's phase 1/2 IT-01 clinical study manuscript for the treatment of metastatic or refractory cancers. The full text article, "Safety and Efficacy of Intratumourally Administered INT230-6 in Adult Patients with Advanced Solid Tumours: Results from an Open-Label Phase 1/2 Dose Escalation Study," can be viewed via Online First 105980 October 29, 2025.
Jacob Stephen Thomas, M.D. Assistant Professor of Clinical Medicine at Keck School of Medicine of the University of Southern California (USC) and medical oncologist with USC's Norris Comprehensive Cancer Center, is the first author. Anthony El-Khoueiry, M.D., Associate Director for Clinical Research and Chief of Section of Developmental Therapeutics/Phase I Program at USC Norris, is the senior and corresponding author.
The manuscript includes the following data results:
- In heavily pretreated patients with advanced disease having over 20 different types of cancer who had progressed following multiple prior lines of therapy, intratumoral INT230-6 achieved:
- A disease control rate of 75% (48/64 patients) and median overall survival (mOS) of 11.9 months; these results compare favorably in phase 1/2 studies that historically reported an mOS of 4 to 7 months
- In a metastatic sarcoma subset population receiving only INT230-6, the median overall survival was 21.3 months
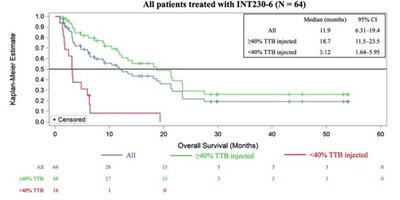
- In an exploratory analysis comparing patients receiving INT230-6 at a total dose (in mL) that treated greater than 40% of the patient's total tumour burden ("TTB") compared to those treated with less than 40% of their TTB, the:
- Disease control rate was 83.3% (40/48) compared to 50% (8/16)
- Median overall survival was 18.7 months (95% CI: 11.5–23.5) compared to 3.1 months (95% CI: 1.6–5.9) with a hazard ratio (HR) of 0.17 (95% CI: 0.081–0.342); P<0.0001 (see Figure 1 below)
- Improved survival was consistent across a range of low to high tumor burden and tumor sizes
- Approximately 20% of patients in the >40% group had uninjected tumors shrink, abscopal effects
- Fifteen of 64 patients survived for more than 21 months
- INT230-6 induced a qualitative decrease in proliferating cancer cells in injected tumors and a qualitative increase in activated T-cells infiltrating the tumor microenvironment
- No dose-limiting toxicities were reported among 64 monotherapy patients; seven patients had a grade 3 (10.9%) with no grade 4 or 5 treatment-related adverse events
- Pharmacokinetic results showed that greater than 95% of the active cytotoxic agents remained in the injected tumors
"INT230-6 is a local treatment that kills cancer using a diffusion process following direct injection into tumors. The trial demonstrated favorable safety and promising efficacy in patients with advanced metastatic cancers who had failed a median of three prior lines of therapy. The disease control rates and median survival compare favorably to those historically seen for such a diverse set of refractory cancer types in a phase 1/2 study," said Jacob S. Thomas, M.D. "There were also several learnings about INT230-6 dosing and safety gained during this trial. The pharmacokinetic data indicated that high rates of the drug are absorbed by the injected tumor, with minimal leakage, even at doses as high as 175 mL administered to a single tumor. These results are consistent with the low incidence of grade 3 adverse events observed."
"The mechanism by which cancer is killed through the diffusion of cytotoxic agents following intratumoral injection of INT230-6 and systemic immune activation, as observed in preclinical models, translated well in the human setting. Uninjected tumors shrinking from a locally administered therapy, referred to as abscopal effects, are generally rare for local therapies. Yet, an abscopal effect was observed in at least 20% of 48 patients who received drug volumes above 40% of their tumor burden. In addition, in thirteen of fourteen matched pair biopsy slides, a notable increase in activated CD4+ and CD8+ T cells was observed in the tumor microenvironment in response to INT230-6 treatment. Representative images can be found in the paper," said Anthony El-Khoueiry M.D. "The abscopal effects and immune cell infiltration observed in this study highlight this intratumoral therapy's potential to drive both a local and systemic anti-cancer activity."
"This comprehensive paper is the culmination of over a decade of nonclinical and clinical research. The article describes the development of a new technology to destroy tumors using molecular agents that can disperse potent anti-cancer compounds within injected tumors and deliver them into cancer cells. We believe these are the first clinical results where a locally administered therapy used alone could potentially extend survival for patients with metastatic disease," said Lewis H. Bender, Founder, President, and CEO of Intensity Therapeutics, Inc. "As Drs. Thomas and El-Khoueiry noted, our paper reports that INT230-6 injected into visible tumors in metastatic patients at an amount based on the size of the injected tumors supports the hypothesis that INT230-6 causes immunologic cancer cell death, even in cancers that are considered immunologically cold. Given the drug's mechanism of action and the data reported in this paper from over 20 types of metastatic solid cancers, such as breast, sarcoma, pancreatic, lung, and head and neck, we believe the study results show the potential of INT230-6 to achieve clinical benefit for metastatic patients of multiple cancer types with or without the use of radiation, systemic drugs or immunotherapy. As a result, we have initiated randomized controlled studies, including a Phase 3 study in sarcoma (NCT06263231)."
The Company will be hosting a conference call featuring two key authors of the study on Friday, October 31, 2025 at 9:00AM ET to discuss the results. Interested parties can access the call by clicking here: https://event.choruscall.com/mediaframe/webcast.html?webcastid=6uG6ARFf. Participants are encouraged to log on at least 10 minutes prior to the start of the event.
About INT230-6
INT230-6, Intensity's lead proprietary investigational product candidate, is designed for direct intratumoral injection. INT230-6 was discovered using Intensity's proprietary DfuseRx℠ technology platform. The drug consists of two proven, potent anti-cancer agents, cisplatin and vinblastine sulfate, and a diffusion and cell penetration enhancer molecule ("SHAO") that non-covalently conjugates to the two payload drugs, facilitating the dispersion of potent cytotoxic drugs throughout tumors and allowing the active agents to diffuse into cancer cells. These agents remain in the tumor, resulting in a favorable safety profile. In addition to local disease control and direct tumor killing, INT230-6 causes a release of a bolus of neoantigens specific to the malignancy, leading to immune system engagement and systemic anti-tumor effects. Importantly, these effects are mediated without immunosuppression, which often occurs with systemic chemotherapy.
About Study Intensity's Clinical Study IT-01
IT-01 was Intensity's first-in-human, open-label, single-arm phase 1/2 study (NCT03058289) using INT230-6. The study was conducted in patients with advanced, refractory, or metastatic solid tumors at six clinical sites in addition to USC. Other investigators were from Johns Hopkins University, Princess Margaret Hospital in Toronto, Columbia Presbyterian in New York, The Fox Chase Cancer Center in Philadelphia, Houston Methodist, and UMass Memorial. The study was comprised of adults with histologically or cytologically confirmed advanced or metastatic solid tumors who did not respond to or were not candidates for standard therapies and had accessible superficial and/or deep tumors for injection. Dose escalation was achieved by increasing the initial and subsequent total dose volumes (total injected amount), the maximum injected volume per any single tumor, the ratio of drug-volume to tumor-size, the number of injected tumors per session, and the dose frequency (once per month vs. every 2 weeks). Maintenance dosing was added in protocol amendments. A tumor's dose was set as a percentage of the volume of the target tumor, which was calculated from radiologic measurements. There were six monotherapy dose cohorts.
About eBioMedicine
eBioMedicine is a leading open-access translational research journal within the Lancet Discovery Group of journals. eBioMedicine encompasses the spectrum of biomedical research, ranging from preclinical studies with clear human relevance to proof-of-concept, first-in-human studies, and early-phase clinical trials. The journal's editors are dedicated to publishing original research that investigates the basic determinants of human health and disease, the discovery and characterization of new therapeutic targets and treatments, and the identification of biomarkers and diagnostic tools that may help researchers and clinicians better understand and monitor disease. The Journal welcomes studies that elucidate, or aims to modify, disease pathways and mechanisms—to advance knowledge in any biomedical discipline with relevance to human health. The Impact Factor of eBioMedicine is 10.8, according to The Lancet. This indicates that the journal's content is highly cited within the scientific community, particularly within the two years preceding the Journal Citation Reports year.
About Intensity
Intensity is a late-stage clinical biotechnology company whose novel engineered chemistry enables aqueous cytotoxic-containing drug formulations to mix and saturate a tumor's dense, high-fat, pressurized environment following direct intratumoral injection. As a result of the saturation, Intensity's clinical trials have demonstrated the ability of INT230-6 to kill tumors and elicit an adaptive immune response within days of injection, representing a new approach to cancer cell death that holds the potential to shift the treatment paradigm and turn many deadly cancers into chronic diseases even for malignancies that do not respond to conventional immunotherapy. Intensity has completed two clinical studies that enrolled over 200 patients using INT230-6: a Phase 1/2 dose escalation study in metastatic cancers including sarcomas (NCT03058289), and a Phase 2 randomized control clinical trial in locally advanced breast cancer (the "INVINCIBLE-2 Study") (NCT04781725) in women without undergoing chemotherapy prior to their surgery. The Company initiated a Phase 3 trial in soft tissue sarcoma (the "INVINCIBLE-3 Study") (NCT06263231), testing INT230-6 as second or third-line monotherapy compared to the standard of care ("SOC") with overall survival as an endpoint. Intensity also initiated a Phase 2 study in collaboration with The Swiss Group for Clinical Cancer Research, formerly SAKK, now the Swiss Cancer Institute (the "INVINCIBLE-4 Study") (NCT06358573) as part of a Phase 2/3 program evaluating INT230-6 followed by the SOC immunochemotherapy and the SOC alone for patients with presurgical triple-negative breast cancer. Pathological complete response ("pCR") is the endpoint. For more information about Intensity, including publications, papers, and posters about its novel approach to cancer therapeutics, visit www.intensitytherapeutics.com or review our SEC filings.
Forward-Looking Statements
Certain statements in this press release may constitute "forward-looking statements" within the meaning of the United States Private Securities Litigation Reform Act of 1995, as amended to date. These statements include, but are not limited to, statements relating to the Company's expected future plans, cash runway, development activities, projected milestones, business activities or results. When or if used in this communication, the words "may," "could," "should," "anticipate," "believe," "estimate," "expect," "intend," "plan," "predict" and similar expressions and their variants, as they relate to the Company or its management, may identify forward-looking statements. The forward-looking statements contained in this press release are based on management's current expectations and projections about future events. Nevertheless, actual results or events could differ materially from the plans, intentions, and expectations disclosed in, or implied by, the forward-looking statements. These risks and uncertainties, many of which are beyond our control, include: the initiation, timing, progress and results of future preclinical studies and clinical trials and research and development programs; the need to raise additional funding before the Company can expect to generate any revenues from product sales; plans to develop and commercialize product candidates; the timing or likelihood of regulatory filings and approvals; the ability of the Company's research to generate and advance additional product candidates; the risk that product candidates that appear promising in early research and clinical trials do not demonstrate safety and/or efficacy in larger-scale or later clinical trials; the implementation of the Company's business model, strategic plans for the Company's business, product candidates and technology; commercialization, marketing and manufacturing capabilities and strategy; the rate and degree of market acceptance and clinical utility of the Company's system; the Company's competitive position; the Company's intellectual property position; developments and projections relating to the Company's competitors and its industry; the Company's ability to maintain and establish collaborations or obtain additional funding; expectations related to the use of cash and cash equivalents and investments; our potential inability to satisfy the Nasdaq Capital Market's requirements for continued listing and be subject to delisting; estimates regarding expenses, future revenue, capital requirements and needs for additional financing; and other risks described in the section entitled "Risk Factors" in the Company's Annual Report on Form 10-K for the year ended December 31, 2024 and in the Company's subsequent SEC filings, which can be obtained on the SEC website at www.sec.gov. Readers are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date on which they are made and reflect management's current estimates, projections, expectations and beliefs. The Company does not plan to update any such forward-looking statements and expressly disclaims any duty to update the information contained in this press release except as required by law.
Investor Relations Contact:
Justin Kulik
Justin@coreir.com
CORE IR
(516) 222-2560
Media Contact:
Matt Cossel
CORE IR
pr@coreir.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/intensity-therapeutics-inc-announces-publication-of-clinical-results-of-int230-6-for-the-treatment-of-metastatic-or-refractory-cancers-in-ebiomedicine-a-lancet-discovery-science-journal-302599519.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/intensity-therapeutics-inc-announces-publication-of-clinical-results-of-int230-6-for-the-treatment-of-metastatic-or-refractory-cancers-in-ebiomedicine-a-lancet-discovery-science-journal-302599519.html
SOURCE Intensity Therapeutics Inc.
Released October 30, 2025