

October 2021 A NEW WEAPON IN THE WAR ON CANCER Directly killing tumors to activate a patient - specific immune response Treating all stages of cancer April 2022 Issuer Free Writing Prospectus Filed pursuant to Rule 433 Registration Number 333 - 260565 Novel, Highly Tumor Diffusive Cytotoxic Drug Products Dosed into Tumors

Intensity Therapeutics, Inc. (the “Company” or “we”) has filed a registration statement, including a preliminary prospectus, with the U.S. Securities and Exchange Commission (the “SEC”) (File No. 333 - 260565) in connection with the offering to which this presentation relates. Sales of the securities of the Company offered pursuant to the registration statement may not be made or offers for such securities accepted prior to the registration statement becoming effective. Before you invest, you should read the registration statement, the preliminary prospectus included within the registration statement and other documents the Company has filed with the SEC for more complete information about the Company and this offering. You can obtain a copy of the preliminary prospectus for free by visiting EDGAR on the SEC website at www.sec.gov. Alternatively, the Company will arrange to send you the preliminary prospectus, which you may request by emailing jwesolowski@intensitytherapeutics.com. This presentation may not be reproduced, forwarded to any person or published, in whole or in part. The Company is not soliciting offers to buy securities of the Company in any jurisdiction where the offer or sale is not permitted. This presentation contains forward - looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties, including statements regarding the development and regulatory status of our product candidates, such as statements with respect to our lead product candidate INT230 - 6, and the timing of clinical trials and data from those trials for our product candidates, and our discovery programs that may lead to our development of additional product candidates, the potential utility of our technology and therapeutic potential of our product candidates, the potential commercialization of any of our product candidates, and the sufficiency of our cash resources. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward - looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward - looking statements, although not all forward - looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward - looking statements, and you should not place undue reliance on our forward - looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward - looking statements we make as a result of various risks and uncertainties, including but not limited to: whether we will be able to successfully conduct Phase 1, 2 or 3 clinical trials for INT230 - 6, whether we complete other clinical trials for our product candidates, whether we receive results from our clinical trials on our expected timelines, or at all, whether our cash resources will be sufficient to fund our foreseeable and unforeseeable operating expenses and capital expenditure requirements on our expected timeline, whether the COVID - 19 pandemic impacts our operations, and other factors included in the “Risk Factors” section of the Company’s filings with the SEC in the future. Any of these outcomes could cause our actual results to differ from those contained in the forward - looking statements of the Company’s filings with the SEC. The forward - looking statements contained in this presentation reflect our current views as of the date of this presentation with respect to future events, and we assume no obligation to update any forward - looking statements except as required by applicable law. The Intensity Therapeutics, Inc. name and logo are our trademarks. We also own the service mark and the registered U.S. trademark for DfuseRx. The trademarks, trade names and service marks appearing in this presentation are the property of the Company. We have omitted the ® and Ρ designations, as applicable, for the trademarks named in 2 this presentation. SAFE HARBOR AND FORWARD - LOOKING STATEMENTS
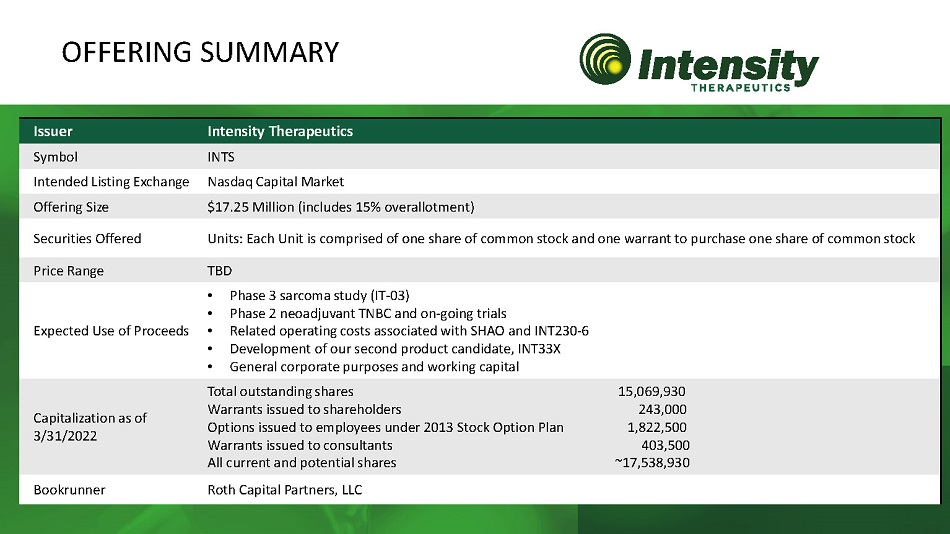
Issuer Intensity Therapeutics Symbol INTS Intended Listing Exchange Nasdaq Capital Market Offering Size $17.25 Million (includes 15% overallotment) Securities Offered Units comprised of one share of common stock and one warrant to purchase one share of common stock Price Range TBD Expected Use of Proceeds • Phase 3 sarcoma study (IT - 03) • Phase 2 neoadjuvant TNBC and on - going trials • Related operating costs associated with SHAO and INT230 - 6 • Development of our second product candidate, INT33X • General corporate purposes and working capital Capitalization as of 3/31/2022 Total outstanding shares 15 , 069 , 930 Warrants issued to shareholders 243 , 000 Options issued to employees under 2013 Stock Option Plan 1 , 822 , 500 Warrants issued to consultants 403 , 500 All current and potential shares ~ 17 , 538 , 930 Bookrunner Roth Capital Partners, LLC OFFERING SUMMARY

4 MANAGEMENT TEAM: EXTENSIVE ONCOLOGY AND DRUG DEVELOPMENT EXPERIENCE Lewis H. Bender , MIT ChE, MS, MA, MBA • CEO, CTO, VP, BD & Manufacturing: Emisphere • CEO: Genomic testing, Interleukin Genetics • Roche, Manufacturing • Drug delivery expertise Preclinical through Phase 3 • Public biotech company CEO experience Ian B. Walters , MD, MBA • Clinical Development 30+ compounds: BMS, Millennium, PDL, Rockefeller University • Translational Medicine: Rockefeller At BMS 7+ years: Oversaw oncology protocol review, and IO clin James M. Ahlers • Danforth Advisors • Intarcia Therapeutics, CFO • 25 years, multiple transactions • Titan Pharmaceutics, IPO Steve Innaimo Bristol - Myers Squibb Rebecca Drain Bristol - Myers Squibb John Wesolowski, MBA, CPA Yale, KMG Main Hurdman BOARD OF DIRECTORS Declan Doogan, Ph.D. Former VP Development Pfizer Emer Leahy, Ph.D. CEO Psychogenics Mark A. Goldberg, MD Former President & COO of PAREXEL Lewis H. Bender CEO Intensity Founder, CEO Chief Medical Officer Principal Accounting Officer and Controller Chief Financial Officer VP, Regulatory & Quality VP, Project Management Executive VP, Clinical Development Brian Schwartz, MD • Mereo • Arqule • Ziopharm • Life Sci

Localized Cancer Kill Leading to Immune Activation and Extended Survival • Novel product candidate (INT230 - 6) containing cytotoxic agents with a diffusion enhancing molecule • Favorable safety with efficacy; 168 patients treated through March 31, 2022, • No maximum tolerated dose; adverse events are mostly low grade; >95% of drug remaining in tumor Multiple Phase 2 Studies ongoing; Phase 3 Registration Sarcoma Study Designed – FDA alignment On Protocol • Regulatory path to approval in sarcoma and triple negative breast cancer (TNBC); FDA Fast Track designation granted for TNBC Robust IP Position • US Patents: 3 issued, 1 pending: 10,888,618; 9,636,406; 9,351, 997: 12 issued foreign patents (4 pending) 100% owned by Intensity (INTS) Platform Validated Through Partnerships • Awarded CRADA by the National Cancer Institute (NCI) • Clinical trial with Ottawa Hospital & Ontario Institute of Cancer Research • Clinical collaborations with world leading immunotherapies Merck’s Keytruda® & Bristol - Myers Squibb’s Yervoy ® 5 (INTS) INVESTMENT HIGHLIGHTS
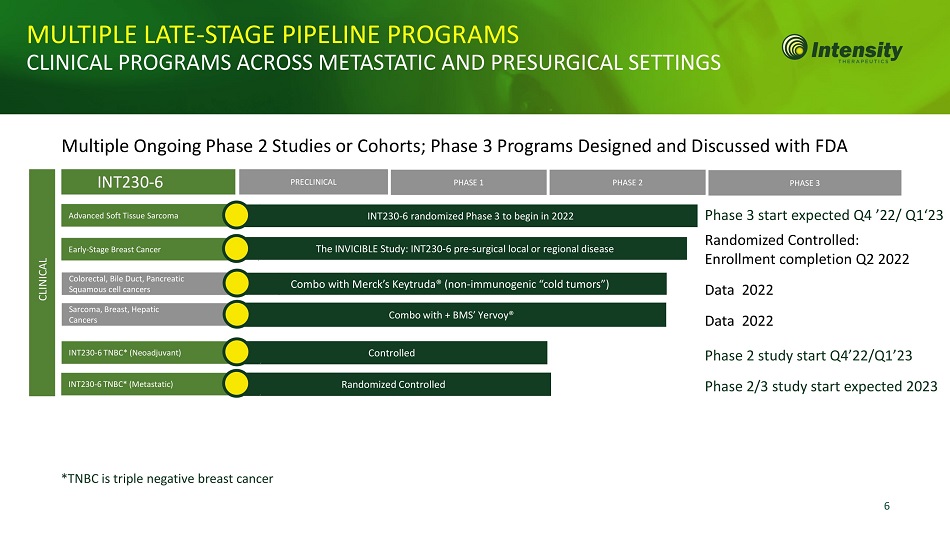
6 INT230 - 6 PRECLINICAL PHASE 1 PHASE 2 PHASE 3 Colorectal, Bile Duct, Pancreatic Squamous cell cancers Sarcoma, Breast, Hepatic Cancers Combo with + BMS’ Yervoy® Combo with Merck’s Keytruda® (non - immunogenic “cold tumors”) Controlled INT230 - 6 TNBC* (Neoadjuvant) MULTIPLE LATE - STAGE PIPELINE PROGRAMS CLINICAL PROGRAMS ACROSS METASTATIC AND PRESURGICAL SETTINGS Multiple Ongoing Phase 2 Studies or Cohorts; Phase 3 Programs Designed and Discussed with FDA The INVICIBLE Study: INT230 - 6 pre - surgical local or regional disease Advanced Soft Tissue Sarcoma INT230 - 6 randomized Phase 3 to begin in 2022 Early - Stage Breast Cancer CLINICAL Phase 3 start expected Q4 ’22/ Q1‘23 Randomized Controlled: Enrollment completion Q2 2022 Data 2022 Data 2022 Phase 2 study start Q4’22/Q1’23 Phase 2/3 study start expected 2023 *TNBC is triple negative breast cancer Randomized Controlled INT230 - 6 TNBC* (Metastatic)
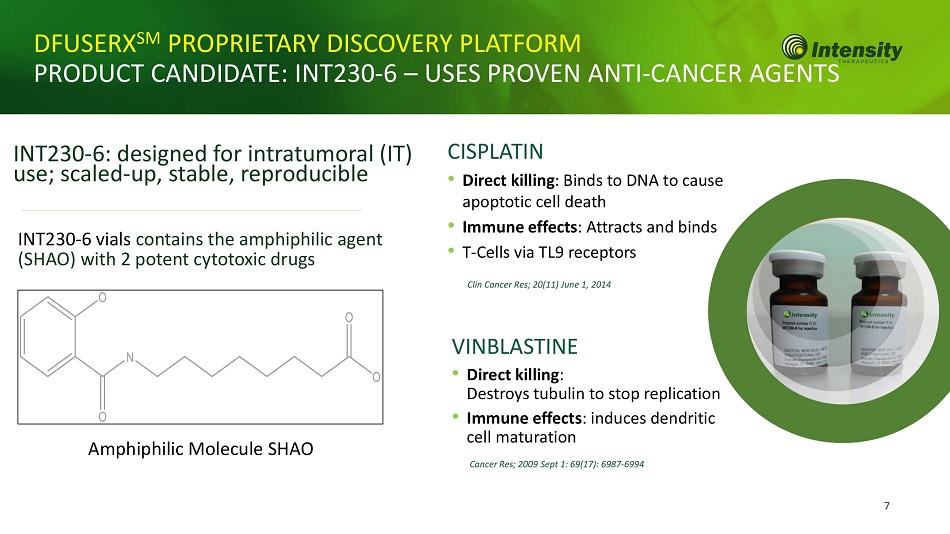
7 DFUSERX SM PROPRIETARY DISCOVERY PLATFORM PRODUCT CANDIDATE: INT230 - 6 – USES PROVEN ANTI - CANCER AGENTS INT230 - 6: designed for intratumoral (IT) use; scaled - up, stable, reproducible INT230 - 6 vials contains the amphiphilic agent (SHAO) with 2 potent cytotoxic drugs Amphiphilic Molecule SHAO CISPLATIN • Direct killing : Binds to DNA to cause apoptotic cell death • Immune effects : Attracts and binds • T - Cells via TL9 receptors Clin Cancer Res; 20(11) June 1, 2014 VINBLASTINE • Direct killing : Destroys tubulin to stop replication • Immune effects : induces dendritic cell maturation Cancer Res; 2009 Sept 1: 69(17): 6987 - 6994
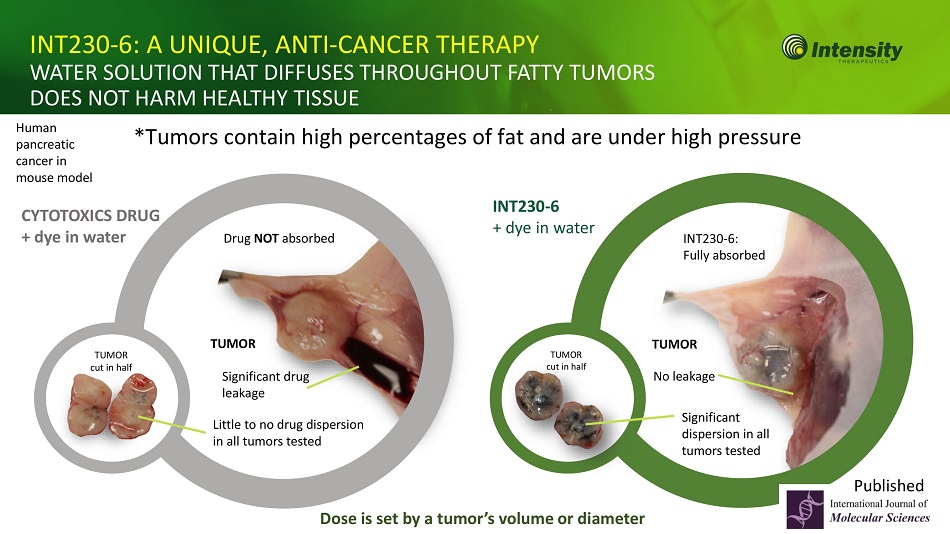
8 INT230 - 6: A UNIQUE, ANTI - CANCER THERAPY WATER SOLUTION THAT DIFFUSES THROUGHOUT FATTY TUMORS DOES NOT HARM HEALTHY TISSUE *Tumors contain high percentages of fat and are under high pressure CYTOTOXICS DRUG + dye in water Little to no drug dispersion in all tumors tested TUMOR cut in half Significant drug leakage Drug NOT absorbed Human pancreatic cancer in mouse model TUMOR cut in half Significant dispersion in all tumors tested No leakage INT230 - 6: Fully absorbed INT230 - 6 + dye in water Dose is set by a tumor’s volume or diameter TUMOR TUMOR Published
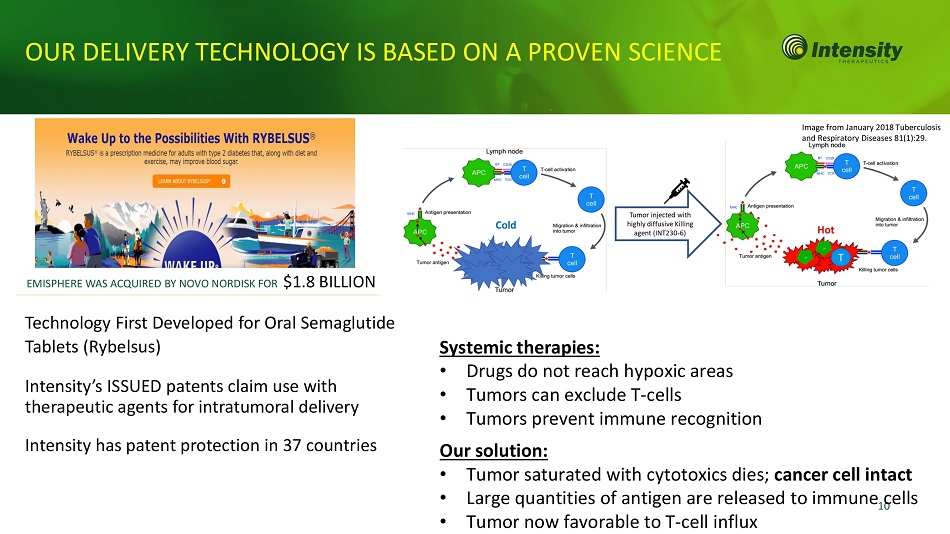
OUR DELIVERY TECHNOLOGY IS BASED ON A PROVEN SCIENCE Technology First Developed for Oral Semaglutide Tablets (Rybelsus) Intensity’s ISSUED patents claim use with therapeutic agents for intratumoral delivery Intensity has patent protection in 37 countries EMISPHERE WAS ACQUIRED BY NOVO NORDISK FOR $1.8 BILLION Systemic therapies: • Drugs do not reach hypoxic areas • Tumors can exclude T - cells • Tumors prevent immune recognition Our solution: • Tumor saturated with cytotoxics dies; cancer cell intact • Large quantities of antigen are released to i m m un e 1 c 0 ells • Tumor now favorable to T - cell influx Image from January 2018 Tuberculosis and Respiratory Diseases 81(1):29.
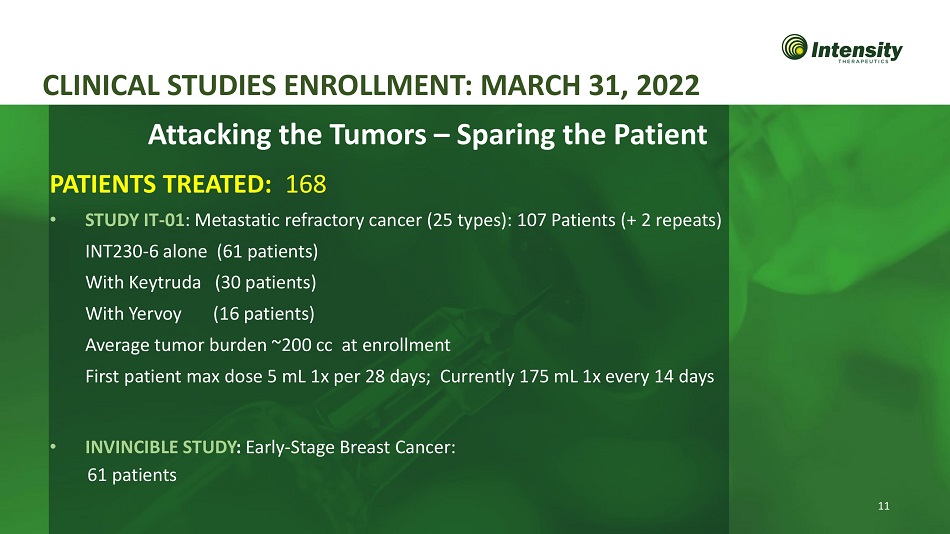
11 CLINICAL STUDIES ENROLLMENT: MARCH 31, 2022 • STUDY IT - 01 : Metastatic refractory cancer (25 types): 107 Patients (+ 2 repeats) INT230 - 6 alone (61 patients) With Keytruda (30 patients) With Yervoy (16 patients) Average tumor burden ~200 cc at enrollment First patient max dose 5 mL 1x per 28 days; Currently 175 mL 1x every 14 days • INVINCIBLE STUDY : Early - Stage Breast Cancer: 61 patients Attacking the Tumors – Sparing the Patient PATIENTS TREATED: 168
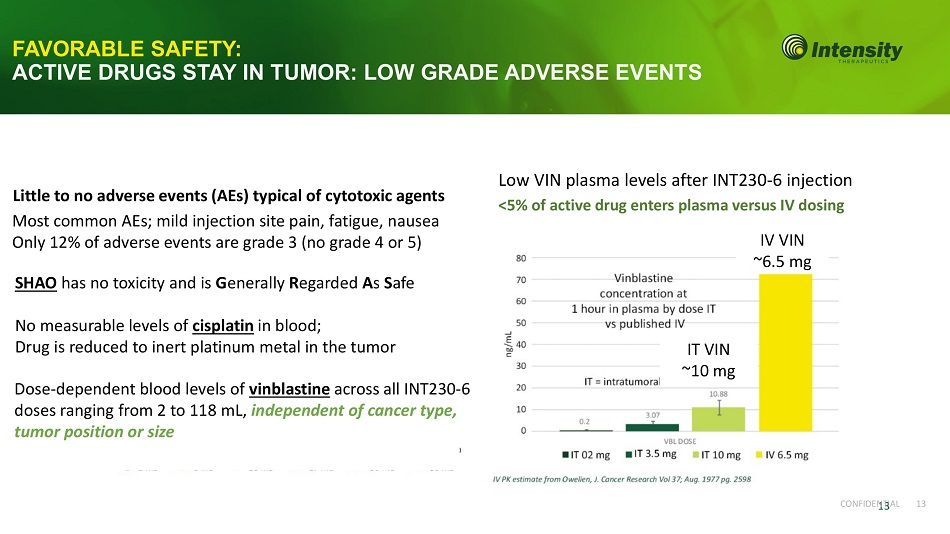
C ON F I D E N 1 T 3 I A L 13 FAVORABLE SAFETY: ACTIVE DRUGS STAY IN TUMOR: LOW GRADE ADVERSE EVENTS IV VIN ~6.5 mg Dose - dependent blood levels of vinblastine across all INT230 - 6 doses ranging from 2 to 118 mL, independent of cancer type, tumor position or size SHAO has no toxicity and is G enerally R egarded A s S afe No measurable levels of cisplatin in blood; Drug is reduced to inert platinum metal in the tumor Low VIN plasma levels after INT230 - 6 injection <5% of active drug enters plasma versus IV dosing IT VIN ~10 mg Little to no adverse events (AEs) typical of cytotoxic agents Most common AEs; mild injection site pain, fatigue, nausea Only 12% of adverse events are grade 3 (no grade 4 or 5)
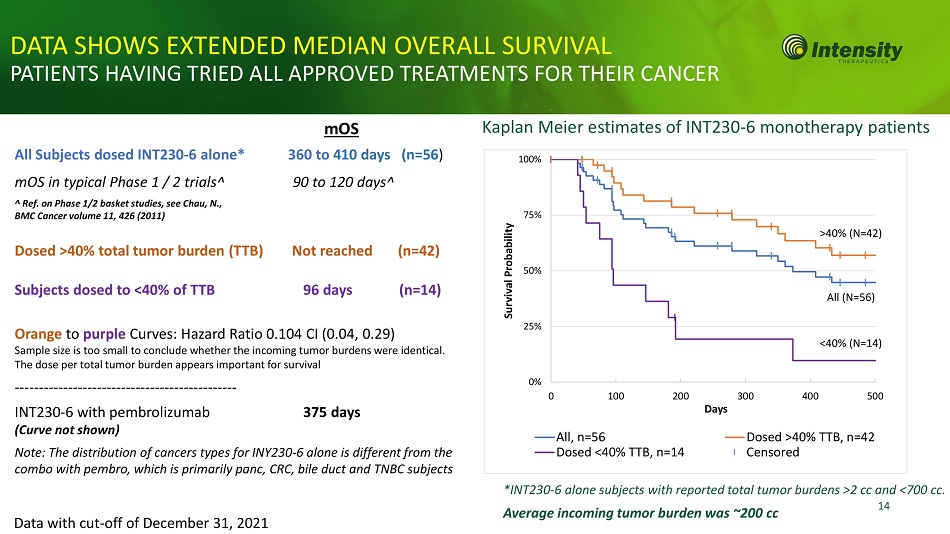
*INT230 - 6 alone subjects with reported total tumor burdens >2 cc and <700 cc. 14 0% 25% 50% 75% 100% 0 100 200 300 400 500 Survival Probability Days All, n=56 Dosed <40% TTB, n=14 Dosed >40% TTB, n=42 Censored <40% (N=14) DATA SHOWS EXTENDED MEDIAN OVERALL SURVIVAL PATIENTS HAVING TRIED ALL APPROVED TREATMENTS FOR THEIR CANCER Kaplan Meier estimates of INT230 - 6 monotherapy patients Data with cut - off of December 31, 2021 mOS 360 to 410 days (n=56 ) 90 to 120 days^ All Subjects dosed INT230 - 6 alone* mOS in typical Phase 1 / 2 trials^ ^ Ref. on Phase 1/2 basket studies, see Chau, N., BMC Cancer volume 11, 426 (2011) Dosed >40% total tumor burden (TTB) Not reached (n=42) Subjects dosed to <40% of TTB 96 days (n=14) Orange to purple Curves: Hazard Ratio 0.104 CI (0.04, 0.29) Sample size is too small to conclude whether the incoming tumor burdens were identical. The dose per total tumor burden appears important for survival --------- - - - -- - -- - - - -- - -- - -- - - - -- - -- - - - -- - -- - - INT230 - 6 with pembrolizumab 375 days (Curve not shown) Note: The distribution of cancers types for INY230 - 6 alone is different from the combo with pembro, which is primarily panc, CRC, bile duct and TNBC subjects Average incoming tumor burden was ~200 cc >40% (N=42) All (N=56)
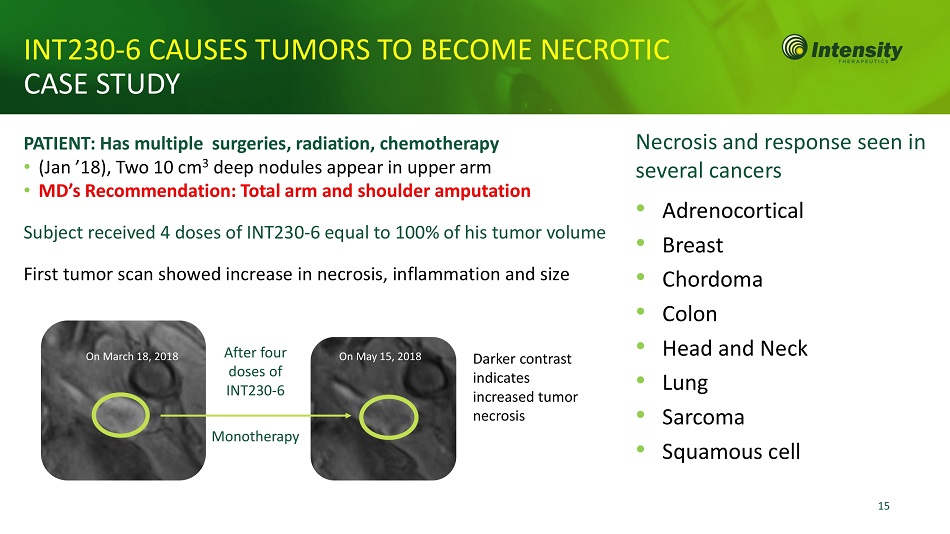
15 INT230 - 6 CAUSES TUMORS TO BECOME NECROTIC CASE STUDY PATIENT: Has multiple surgeries, radiation, chemotherapy • (Jan ’18), Two 10 cm 3 deep nodules appear in upper arm • MD’s Recommendation: Total arm and shoulder amputation Subject received 4 doses of INT230 - 6 equal to 100% of his tumor volume First tumor scan showed increase in necrosis, inflammation and size On March 18, 2018 On May 15, 2018 After four doses of INT 230 - 6 Monotherapy Necrosis and response seen in several cancers • Adrenocortical • Breast • Chordoma • Colon • Head and Neck • Lung • Sarcoma • Squamous cell Darker contrast indicates increased tumor necrosis
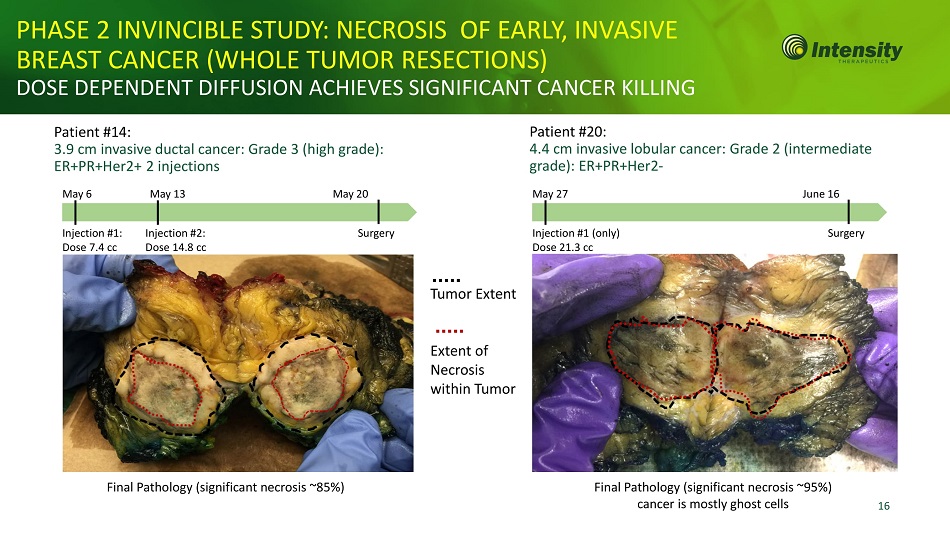
16 PHASE 2 INVINCIBLE STUDY: NECROSIS OF EARLY, INVASIVE BREAST CANCER (WHOLE TUMOR RESECTIONS) DOSE DEPENDENT DIFFUSION ACHIEVES SIGNIFICANT CANCER KILLING Patient #14: 3.9 cm invasive ductal cancer: Grade 3 (high grade): ER+PR+Her2+ 2 injections May 6 May 13 May 20 Final Pathology (significant necrosis ~85%) Patient #20: 4.4 cm invasive lobular cancer: Grade 2 (intermediate grade): ER+PR+Her2 - May 27 June 16 Final Pathology (significant necrosis ~95%) cancer is mostly ghost cells Injection #1: Dose 7.4 cc Injection #2: Dose 14.8 cc Surgery Injection #1 (only) Dose 21.3 cc Surgery Tumor Extent Extent of Necrosis within Tumor
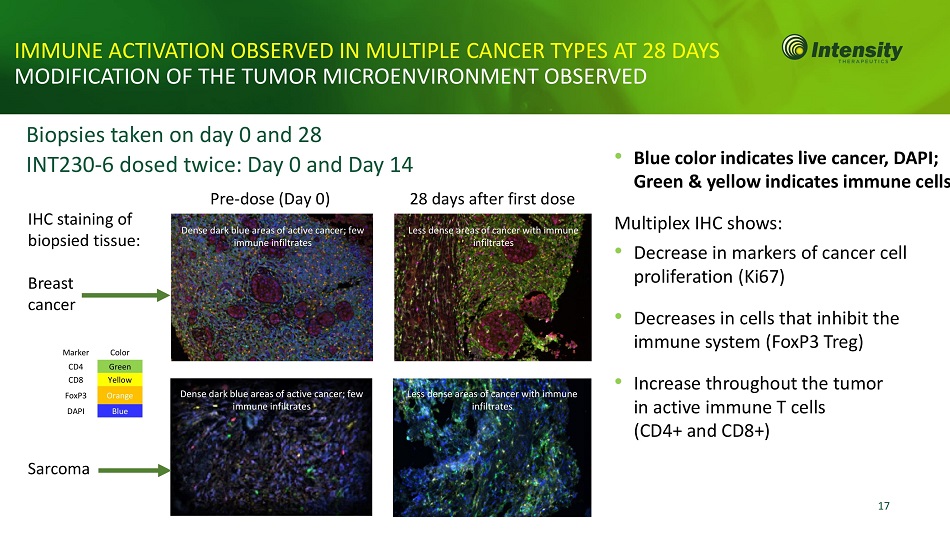
17 IMMUNE ACTIVATION OBSERVED IN MULTIPLE CANCER TYPES AT 28 DAYS MODIFICATION OF THE TUMOR MICROENVIRONMENT OBSERVED Biopsies taken on day 0 and 28 INT230 - 6 dosed twice: Day 0 and Day 14 • Blue color indicates live cancer, DAPI; Green & yellow indicates immune cells Multiplex IHC shows: • Decrease in markers of cancer cell proliferation (Ki67) • Decreases in cells that inhibit the immune system (FoxP3 Treg) • Increase throughout the tumor in active immune T cells (CD4+ and CD8+) IHC staining of biopsied tissue: Breast cancer Sarcoma Pre - dose (Day 0) 28 days after first dose Marker Color CD4 Green CD8 Yellow FoxP3 Orange DAPI Blue Dense dark blue areas of active cancer; few immune infiltrates Less dense areas of cancer with immune infiltrates Dense dark blue areas of active cancer; few immune infiltrates Less dense areas of cancer with immune infiltrates
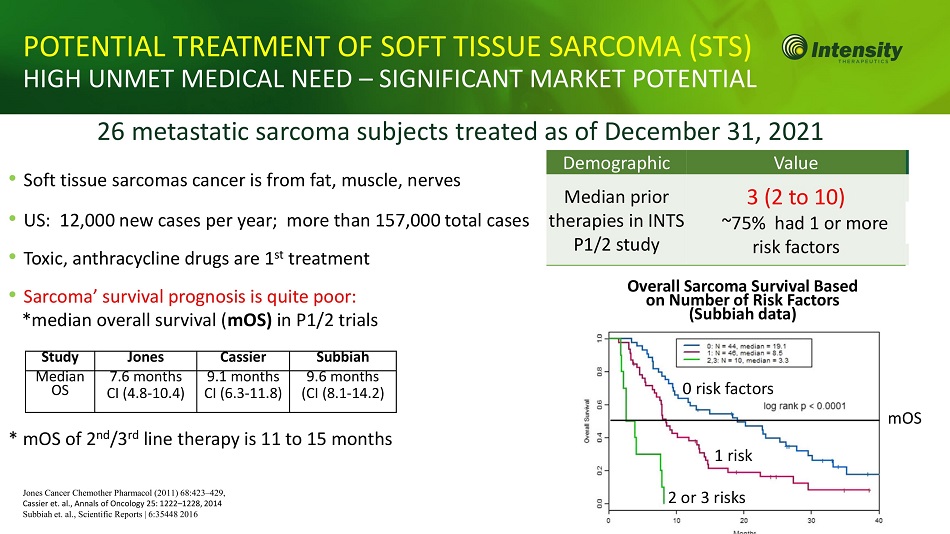
18 POTENTIAL TREATMENT OF SOFT TISSUE SARCOMA (STS) HIGH UNMET MEDICAL NEED – SIGNIFICANT MARKET POTENTIAL 26 metastatic sarcoma subjects treated as of December 31, 2021 • Soft tissue sarcomas cancer is from fat, muscle, nerves • US: 12,000 new cases per year; more than 157,000 total cases • Toxic, anthracycline drugs are 1 st treatment • Sarcoma’ survival prognosis is quite poor: *median overall survival ( mOS) in P1/2 trials * mOS of 2 nd /3 rd line therapy is 11 to 15 months Demographic Value Median prior therapies in INTS P1/2 study 3 (2 to 10) ~75% had 1 or more risk factors Study Jones Cassier Subbiah Median OS 7.6 months CI (4.8 - 10.4) 9.1 months CI (6.3 - 11.8) 9.6 months (CI (8.1 - 14.2) Overall Sarcoma Survival Based on Number of Risk Factors (Subbiah data) 2 or 3 risks 1 risk 0 risk factors mOS Jones Cancer Chemother Pharmacol (2011) 68:423 – 429, Cassier et. al., Annals of Oncology 25: 1222 – 1228, 2014 Subbiah et. al., Scientific Reports | 6:35448 2016
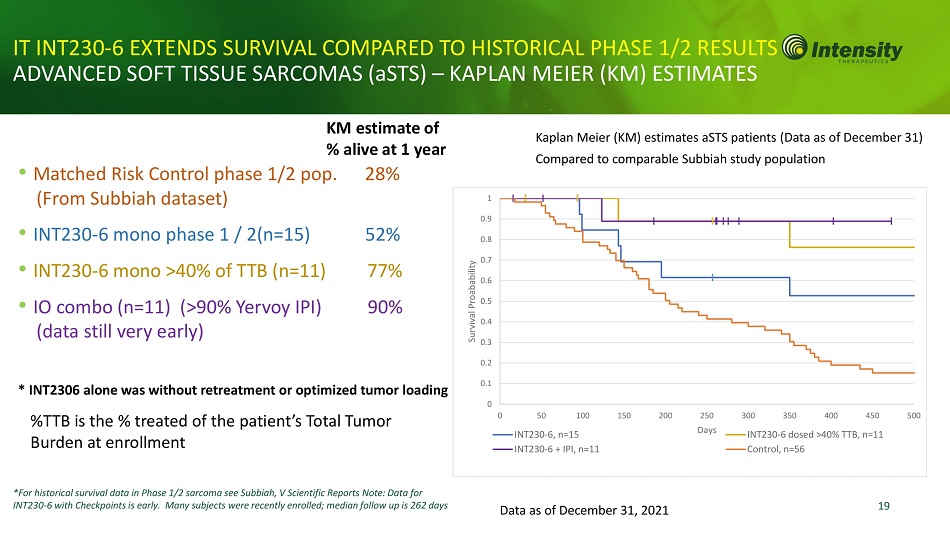
19 • Matched Risk Control phase 1/2 pop. 28% (From Subbiah dataset) • INT230 - 6 mono phase 1 / 2(n=15) 52% • INT230 - 6 mono >40% of TTB (n=11) 77% • IO combo (n=11) (>90% Yervoy IPI) 90% (data still very early) * INT2306 alone was without retreatment or optimized tumor loading IT INT230 - 6 EXTENDS SURVIVAL COMPARED TO HISTORICAL PHASE 1/2 RESUL ADVANCED SOFT TISSUE SARCOMAS (aSTS) – KAPLAN MEIER (KM) ESTIMATES *For historical survival data in Phase 1/2 sarcoma see Subbiah, V Scientific Reports Note: Data for INT230 - 6 with Checkpoints is early. Many subjects were recently enrolled; median follow up is 262 days Data as of December 31, 2021 %TTB is the % treated of the patient’s Total Tumor Burden at enrollment KM estimate of % alive at 1 year Kaplan Meier (KM) estimates aSTS patients (Data as of December 31) Compared to comparable Subbiah study population 1 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 0 150 200 500 Survival Proabability 250 Days 50 100 INT230 - 6, n=15 INT230 - 6 + IPI, n=11 300 350 400 450 INT230 - 6 dosed >40% TTB, n=11 Control, n=56
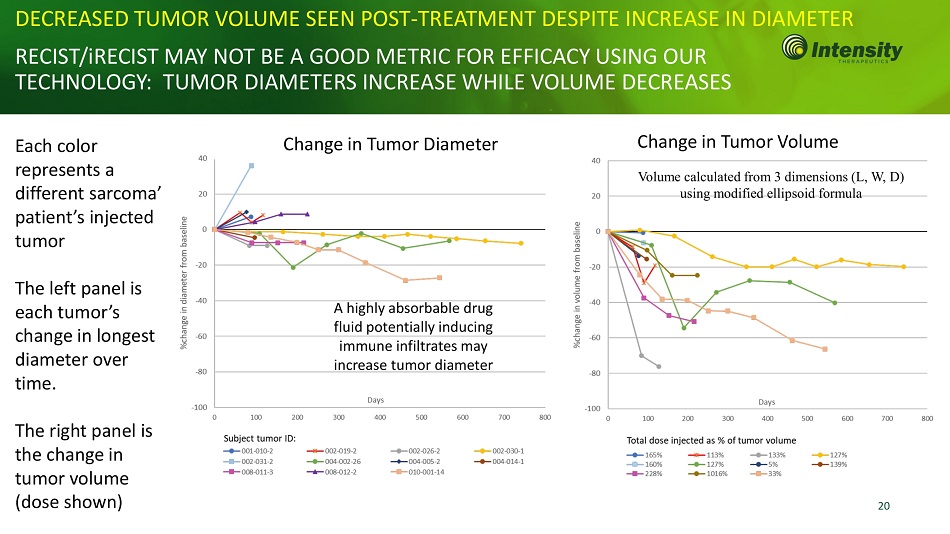
20 DECREASED TUMOR VOLUME SEEN POST - TREATMENT DESPITE INCREASE IN RECIST/iRECIST MAY NOT BE A GOOD METRIC FOR EFFICACY USING OUR TECHNOLOGY: TUMOR DIAMETERS INCREASE WHILE VOLUME DECREASES Each color represents a different sarcoma’ patient’s injected tumor The left panel is each tumor’s change in longest diameter over time. The right panel is the change in tumor volume (dose shown) A highly absorbable drug fluid potentially inducing immune infiltrates may increase tumor diameter Volume calculated from 3 dimensions (L, W, D) using modified ellipsoid formula Change in Tumor Diameter Change in Tumor Volume
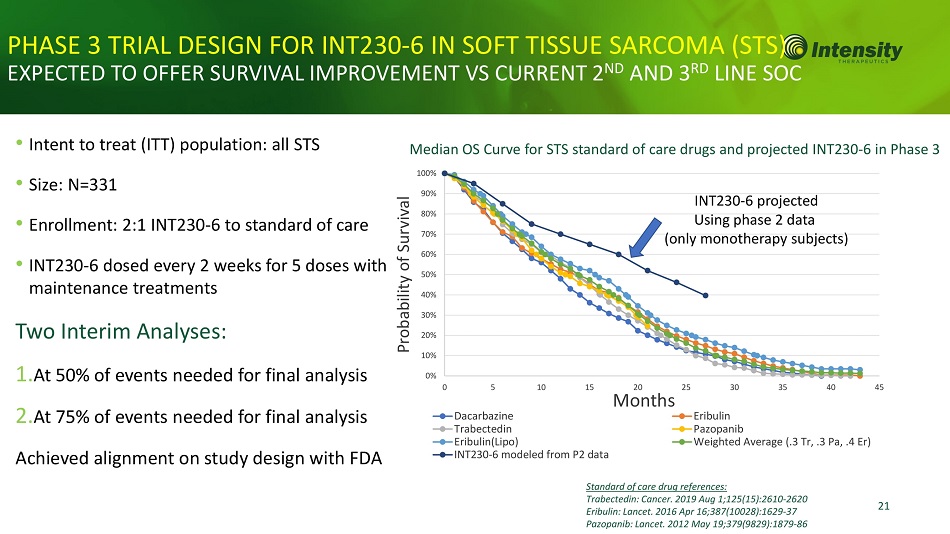
21 PHASE 3 TRIAL DESIGN FOR INT230 - 6 IN SOFT TISSUE SARCOMA (S S) EXPECTED TO OFFER SURVIVAL IMPROVEMENT VS CURRENT 2 ND AND 3 RD LINE SOC Median OS Curve for STS standard of care drugs and projected INT230 - 6 in Phase 3 • Intent to treat (ITT) population: all STS • Size: N=331 • Enrollment: 2:1 INT230 - 6 to standard of care • INT230 - 6 dosed every 2 weeks for 5 doses with maintenance treatments Two Interim Analyses: 1. At 50% of events needed for final analysis 2. At 75% of events needed for final analysis Achieved alignment on study design with FDA Standard of care drug references: Trabectedin: Cancer. 2019 Aug 1;125(15):2610 - 2620 Eribulin: Lancet. 2016 Apr 16;387(10028):1629 - 37 Pazopanib: Lancet. 2012 May 19;379(9829):1879 - 86 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 0 5 10 15 Dacarbazine Trabectedin Eribulin(Lipo) INT 230 - 6 modeled from P 2 data 20 Months 25 30 35 40 Eribulin Pazopanib Weighted Average (.3 Tr, .3 Pa, .4 Er) 45 Probability of Survival INT230 - 6 projected Using phase 2 data (only monotherapy subjects)

22 PLATFORM VALIDATED BY WORLD LEADING PARTNERSHIPS RESEARCH CLINICAL TRIAL SITES

23 MULTIPLE UPCOMING MILESTONES Next 24 months MILESTONES TIMING Multiple data presentations made in Q4 2021; SITC (2) (immunotherapy), CTOS (sarcoma Oral Podium) SABCS (breast cancer) Reported safety and efficacy data of monotherapy and both I/O combinations Q4 2021 Report Phase 2 INVINCIBLE Study data (Part 1) Q2 2022 Report interim IT - 01 data on combination with Keytruda Q2 2022 Report interim IT - 01 data on combination with Yervoy Q2 2022 Initiate randomized Phase 3 international study of INT230 - 6 in the 2 nd /3 rd line sarcoma setting Q4‘22/Q1’23 Report Yervoy and Keytruda Final data 2023 Report Phase 2 Part 2 INVINCIBLE early - stage breast cancer immune and primary efficacy outcome results 1H 2023 Complete Enrollment of Phase 3 Sarcoma Study 2H 2023 Report Phase 2 neoadjuvant TNBC results 1H 2024

24 INTS INVESTMENT SUMMARY A unique and new cancer killing, treatment approach Experienced Oncology Drug Development Management Team Novel Technology to Kill Cancer and Activate the Immune System; Extended Patient Survival Favorable safety; 168 patients as of 03/31/2022 On - going Phase 2 Studies; Phase 3 Sarcoma Registration Study Designed with FDA Alignment Robust IP Position (100% INTS owned) Protection in all Major Markets Platform Validated Through Partnerships Clinical collaborations with world leading organizations

25 INTENSITY THERAPEUTICS A NEW WEAPON IN THE WAR ON CANCER Contact Investor Relations Contact : Rx Communications Group Michael Miller (917) - 633 - 6086 mmiller@rxir.com Thank you!