

October 2021 A NEW WEAPON IN THE WAR ON CANCER Products that directly kill tumors to activate a patient - specific immune response Treating all stages of cancer January 2023 Issuer Free Writing Prospectus Filed pursuant to Rule 433 Registration Number 333 - 260565 Our vision : To extend patient life while maintaining good quality

Intensity Therapeutics, Inc. (the “Company” or “we”) has filed a registration statement, including a preliminary prospectus, with the U.S. Securities and Exchange Commission (the “SEC”) (File No. 333 - 260565) in connection with the offering to which this presentation relates. Sales of the securities of the Company offered pursuant to the registration statement may not be made or offers for such securities accepted prior to the registration statement becoming effective. Before you invest, you should read the registration statement, the preliminary prospectus included within the registration statement and other documents the Company has filed with the SEC for more complete information about the Company and this offering. You can obtain a copy of the preliminary prospectus for free by visiting EDGAR on the SEC website at www.sec.gov. Alternatively, the Company will arrange to send you the preliminary prospectus, which you may request by emailing jwesolowski@intensitytherapeutics.com. This presentation may not be reproduced, forwarded to any person or published, in whole or in part. The Company is not soliciting offers to buy securities of the Company in any jurisdiction where the offer or sale is not permitted. This presentation contains forward - looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties, including statements regarding the development and regulatory status of our product candidates, such as statements with respect to our lead product candidate INT230 - 6, and the timing of clinical trials and data from those trials for our product candidates, and our discovery programs that may lead to our development of additional product candidates, the potential utility of our technology and therapeutic potential of our product candidates, the potential commercialization of any of our product candidates, and the sufficiency of our cash resources. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward - looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward - looking statements, although not all forward - looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward - looking statements, and you should not place undue reliance on our forward - looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward - looking statements we make as a result of various risks and uncertainties, including but not limited to: whether we will be able to successfully conduct Phase 1, 2 or 3 clinical trials for INT230 - 6, whether we complete other clinical trials for our product candidates, whether we receive results from our clinical trials on our expected timelines, or at all, whether our cash resources will be sufficient to fund our foreseeable and unforeseeable operating expenses and capital expenditure requirements on our expected timeline, whether the COVID - 19 pandemic impacts our operations, and other factors included in the “Risk Factors” section of the Company’s filings with the SEC in the future. Any of these outcomes could cause our actual results to differ from those contained in the forward - looking statements of the Company’s filings with the SEC. The forward - looking statements contained in this presentation reflect our current views as of the date of this presentation with respect to future events, and we assume no obligation to update any forward - looking statements except as required by applicable law. The Intensity Therapeutics, Inc. name and logo are our trademarks. We also own the service mark and the registered U.S. trademark for DfuseRx. The trademarks, trade names and service marks appearing in this presentation are the property of the Company. We have omitted the ® and Ρ designations, as applicable, for the trademarks named in 2 this presentation. SAFE HARBOR AND FORWARD - LOOKING STATEMENTS

3 Issuer Intensity Therapeutics Symbol INTS Intended Listing Exchange Nasdaq Capital Market Offering Size 1,777,778 shares based on mid range price of $4.50 (does not include 15% overallotment) Securities Offered Common stock Price Range $4 to $5 per common share Expected Use of Proceeds • Phase 2/3 neoadjuvant TNBC and on - going trials • Phase 3 sarcoma study (IT - 03) • Related operating costs associated with SHAO and INT230 - 6 • Development of our second product candidate, INT33X • General corporate purposes and working capital Capi t ali z a tion as of 11/30/2022 Total outstanding shares 15 , 069 , 930 Warrants issued to shareholders 243 , 000 Options issued to employees under 2013 Stock Option Plan 1 , 702 , 500 W ar r a n ts issu e d t o c onsul t a n ts 403 , 500 All current and potential shares ~ 17 , 418 , 930 (Excluding 4 convertible notes) Bookrunner The Benchmark Company, LLC Co - manager: Joseph Gunnar & Co., LLC OFFERING SUMMARY

4 (INTS) INVESTMENT HIGHLIGHTS Localized Cancer Kill Leading to Immune Activation and Extended Survival • Favorable safety with efficacy; over 200 patients enrolled Phase 2 Studies Finishing; Phase 3 Registration Studies Designed – FDA alignment On Protocol • FDA Fast Track designation granted for TNBC; Orphan drug designation for soft tissue arcoma Robust IP Position • Multiple US Patents: 12 issued foreign patents 100% owned by Intensity (INTS ) Platform Validated Through R&D Partnerships

5 PLATFORM VALIDATED BY WORLD LEADING PARTNERS RESEARCH CLINICAL TRIAL SITES

6 MANAGEMENT TEAM: EXTENSIVE ONCOLOGY AND DRUG DEVELOPMENT EXPERIENCE Lewis H. Bender , MIT ChE, MS, MA, MBA • CEO, CTO, VP, BD & Manufacturing: Emisphere • CEO: Genomic testing, Interleukin Genetics • Roche, Manufacturing • Drug delivery expertise Preclinical through Phase 3 • Public biotech company CEO experience Ian B. Walters , MD, MBA • Clinical Development 30+ compounds: BMS, Millennium, PDL, Rockefeller University • Translational Medicine: Rockefeller At BMS 7+ years: Oversaw oncology protocol review, and IO clin James M. Ahlers • Danforth Advisors • Intarcia Therapeutics, CFO • 25 years, multiple transactions • Titan Pharmaceutics, IPO Steve Innaimo Bristol - Myers Squibb Rebecca Drain Bristol - Myers Squibb John Wesolowski, MBA, CPA Yale, KMG Main Hurdman BOARD OF DIRECTORS Declan Doogan, Ph.D. Former VP Development Pfizer Emer Leahy, Ph.D. CEO Psychogenics Mark A. Goldberg, MD Former President & COO of PAREXEL Lewis H. Bender CEO Intensity F o unde r , CEO Chief M ed i c al Officer Principal Accounting Officer and Controller Chief F i nan ci al Officer Regulatory & Quality VP, Project M ana g eme n t Executive VP, Clinical D ev e l o pme nt Brian Schwartz, MD • M e r eo • Arqule • Zio ph arm • L i f e S c i
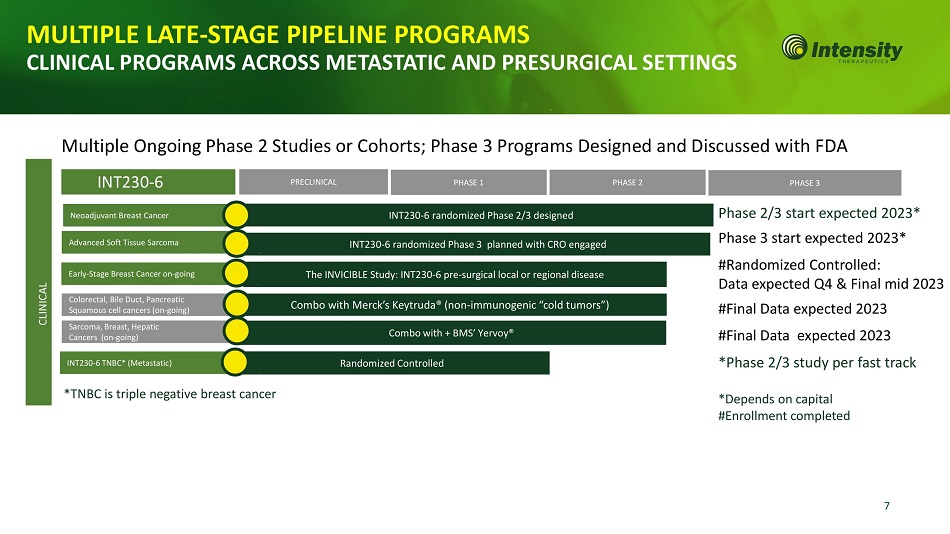
7 Neoadjuvant Breast Cancer INT230 - 6 randomized Phase 2/3 designed INT230 - 6 PRECLINICAL PHASE 1 PHASE 2 PHASE 3 Colorectal, Bile Duct, Pancreatic Squamous cell cancers (on - going) Sarcoma, Breast, Hepatic Cancers (on - going) Combo with + BMS’ Yervoy® Combo with Merck’s Keytruda® (non - immunogenic “cold tumors”) MULTIPLE LATE - STAGE PIPELINE PROGRAMS CLINICAL PROGRAMS ACROSS METASTATIC AND PRESURGICAL SETTINGS Multiple Ongoing Phase 2 Studies or Cohorts; Phase 3 Programs Designed and Discussed with FDA The INVICIBLE Study: INT230 - 6 pre - surgical local or regional disease INT230 - 6 randomized Phase 3 planned with CRO engaged Early - Stage Breast Cancer on - going CLI N IC AL Phase 2/3 start expected 2023* Phase 3 start expected 2023* #Randomized Controlled: Data expected Q4 & Final mid 2023 #Final Data expected 2023 #Final Data expected 2023 *Phase 2/3 study per fast track *Depends on capital #Enrollment completed *TNBC is triple negative breast cancer R and om iz ed C o n t r o ll ed I N T 230 - 6 T N BC* ( Met a st a ti c) Advanced Soft Tissue Sarcoma
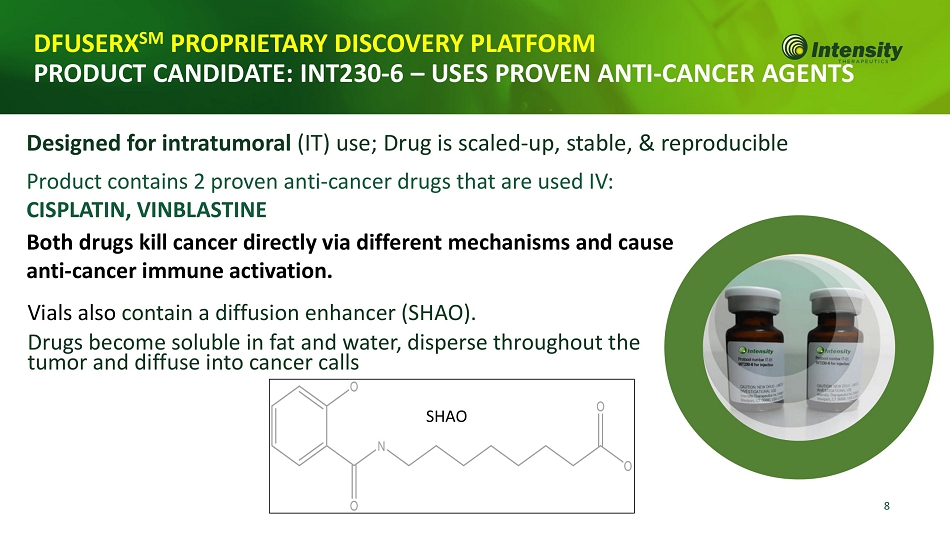
8 DFUSERX SM PROPRIETARY DISCOVERY PLATFORM PRODUCT CANDIDATE: INT230 - 6 – USES PROVEN ANTI - CANCER AGENTS Designed for intratumoral (IT) use; Drug is scaled - up, stable, & reproducible Product contains 2 proven anti - cancer drugs that are used IV: CISPLATIN, VINBLASTINE Both drugs kill cancer directly via different mechanisms and cause anti - cancer immune activation. Vials also contain a diffusion enhancer (SHAO). Drugs become soluble in fat and water, disperse throughout the tumor and diffuse into cancer calls SHAO
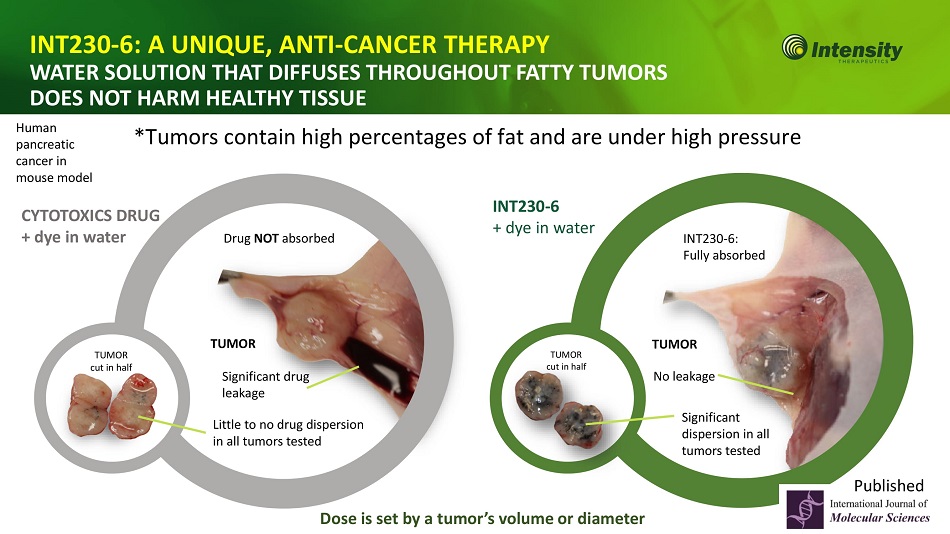
9 INT230 - 6: A UNIQUE, ANTI - CANCER THERAPY WATER SOLUTION THAT DIFFUSES THROUGHOUT FATTY TUMORS DOES NOT HARM HEALTHY TISSUE *Tumors contain high percentages of fat and are under high pressure CYTOTOXICS DRUG + dye in water Little to no drug dispersion in all tumors tested TUMOR cut in half Significant drug leakage Drug NOT absorbed Human pancreatic cancer in mouse model TUMOR cut in half Significant dispersion in all tumors tested No leakage INT230 - 6: Fully absorbed INT230 - 6 + dye in water Dose is set by a tumor’s volume or diameter T UMO R T UMO R Published
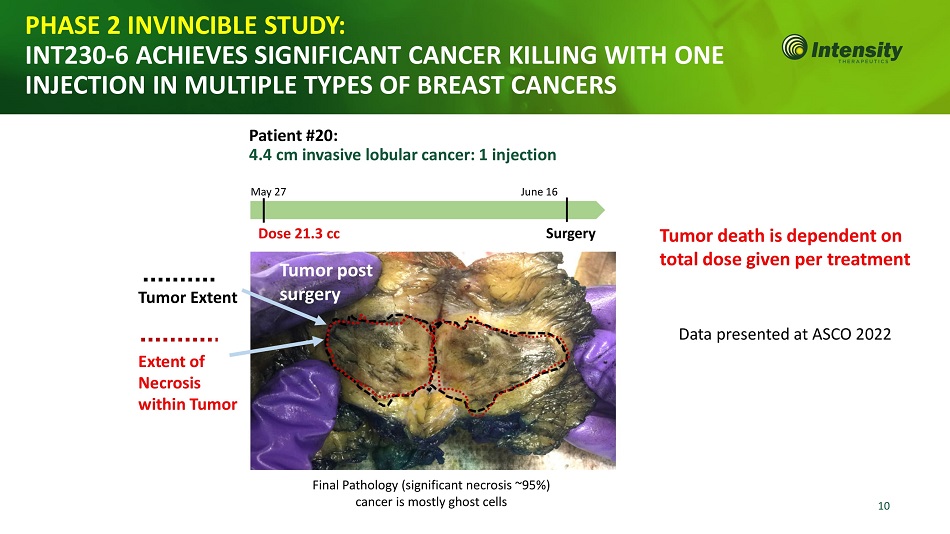
10 Tumor Extent Extent of Necrosis wit h in T u mor PHASE 2 INVINCIBLE STUDY: INT230 - 6 ACHIEVES SIGNIFICANT CANCER KILLING WITH ONE INJECTION IN MULTIPLE TYPES OF BREAST CANCERS Final Pathology (significant necrosis ~95%) cancer is mostly ghost cells Patient #20: 4.4 cm invasive lobular cancer: 1 injection M a y 27 J un e 16 D o s e 21 . 3 cc S u r g e r y T umor po s t surgery Data presented at ASCO 2022 Tumor death is dependent on total dose given per treatment
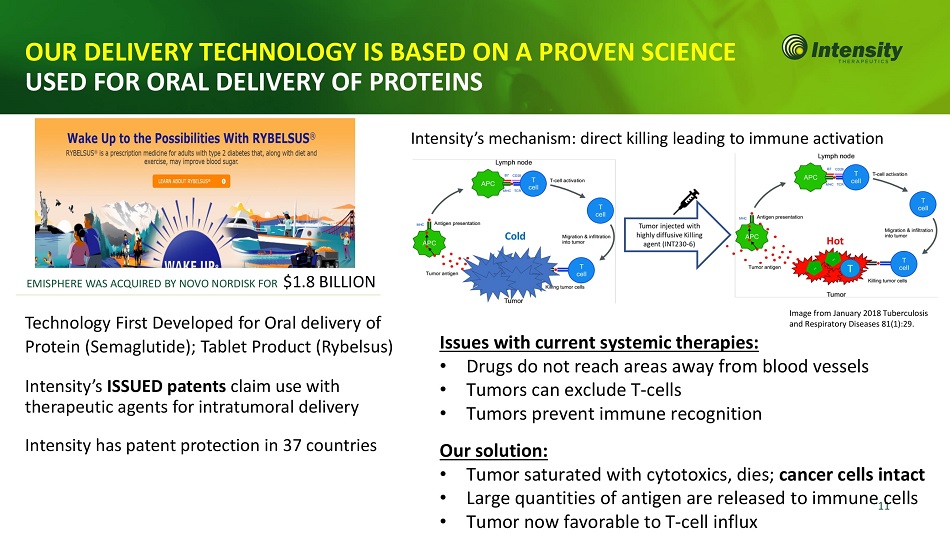
OUR DELIVERY TECHNOLOGY IS BASED ON A PROVEN SCIENCE USED FOR ORAL DELIVERY OF PROTEINS Technology First Developed for Oral delivery of Protein (Semaglutide); Tablet Product (Rybelsus) Intensity’s ISSUED patents claim use with therapeutic agents for intratumoral delivery Intensity has patent protection in 37 countries EMISPHERE WAS ACQUIRED BY NOVO NORDISK FOR $1.8 BILLION Issues with current systemic therapies: • Drugs do not reach areas away from blood vessels • Tumors can exclude T - cells • Tumors prevent immune recognition Our solution: • Tumor saturated with cytotoxics, dies; cancer cells intact • Large quantities of antigen are released to immune 1 c 1 ells • Tumor now favorable to T - cell influx Image from January 2018 Tuberculosis and Respiratory Diseases 81(1):29. Intensity’s mechanism: direct killing leading to immune activation
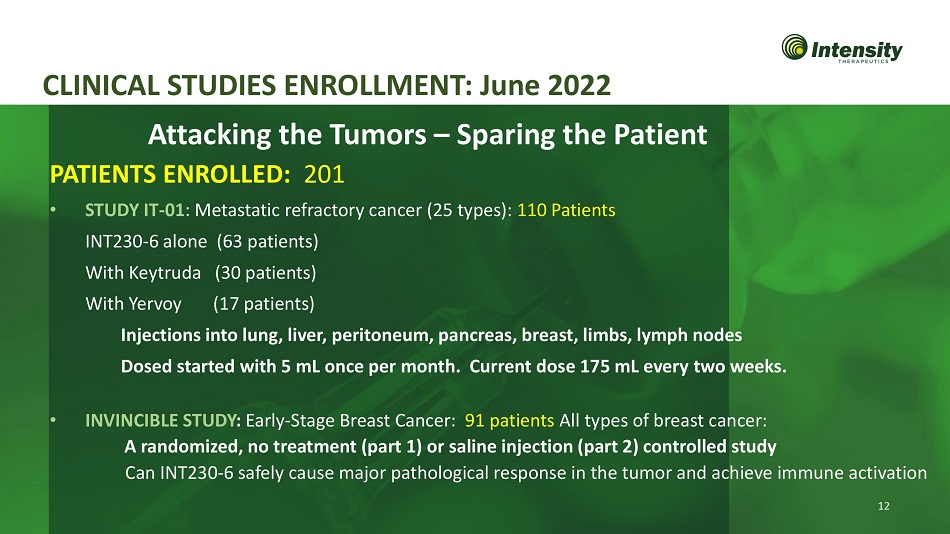
12 CLINICAL STUDIES ENROLLMENT: June 2022 • STUDY IT - 01 : Metastatic refractory cancer (25 types): 110 Patients INT230 - 6 alone (63 patients) With Keytruda (30 patients) With Yervoy (17 patients) Injections into lung, liver, peritoneum, pancreas, breast, limbs, lymph nodes Dosed started with 5 mL once per month. Current dose 175 mL every two weeks. • INVINCIBLE STUDY : Early - Stage Breast Cancer: 91 patients All types of breast cancer: A randomized, no treatment (part 1) or saline injection (part 2) controlled study Can INT230 - 6 safely cause major pathological response in the tumor and achieve immune activation Attacking the Tumors – Sparing the Patient PATIENTS ENROLLED: 201
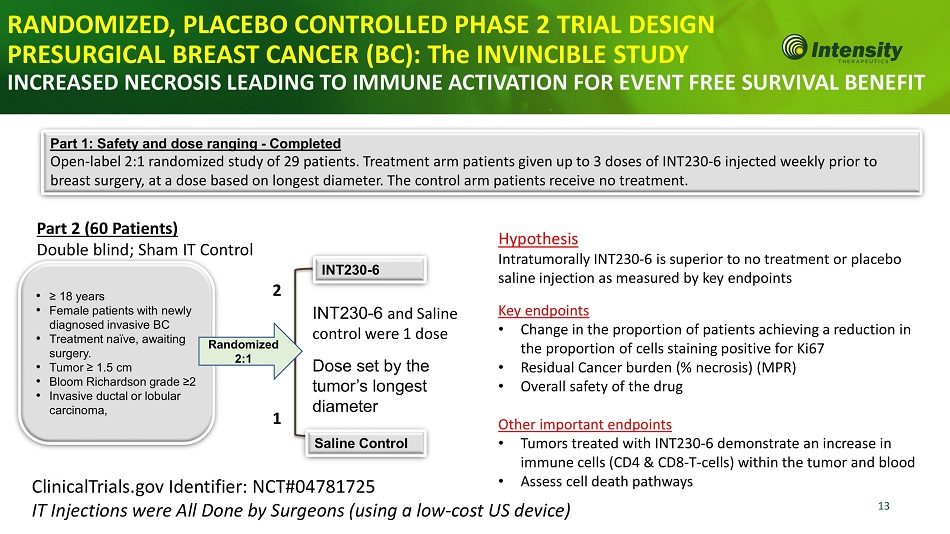
13 • ≥ 18 years • Female patients with newly diagnosed invasive BC • Treatment naïve, awaiting surgery. • Tumor ≥ 1.5 cm • Bloom Richardson grade ≥2 • Invasive ductal or lobular carcinoma, R a ndo mized 2:1 INT230 - 6 Sa li n e Con t r ol Part 1: Safety and dose ranging - Completed Open - label 2:1 randomized study of 29 patients. Treatment arm patients given up to 3 doses of INT230 - 6 injected weekly prior to breast surgery, at a dose based on longest diameter. The control arm patients receive no treatment. ClinicalTrials.gov Identifier: NCT#04781725 IT Injections were All Done by Surgeons (using a low - cost US device) INT230 - 6 and Saline control were 1 dose Dose set by the tumor’s longest diameter Key endpoints • Change in the proportion of patients achieving a reduction in the proportion of cells staining positive for Ki67 • Residual Cancer burden (% necrosis) (MPR) • Overall safety of the drug Other important endpoints • Tumors treated with INT230 - 6 demonstrate an increase in immune cells (CD4 & CD8 - T - cells) within the tumor and blood • Assess cell death pathways RANDOMIZED, PLACEBO CONTROLLED PHASE 2 TRIAL DESIGN PRESURGICAL BREAST CANCER (BC): The INVINCIBLE STUDY INCREASED NECROSIS LEADING TO IMMUNE ACTIVATION FOR EVENT FREE SURVIVAL BENEFIT Part 2 (60 Patients) Double blind; Sham IT Control 2 1 Hypothesis Intratumorally INT230 - 6 is superior to no treatment or placebo saline injection as measured by key endpoints

14 NEOADJUVANT STUDY SAFETY INT230 - 6 HAD FAVORABLE SAFETY • No surgery was delayed or cancelled • No surgical procedure was altered • No cosmetic differences noted • Mean wait time to surgery: 24 days (range 14 - 34 days) – normal timeframe • 89% of adverse events were grade 1; all resolved within 7 days • Patient interest in the drug and acceptability was high, accrual was rapid Pre vs. Post treatment (part1) • In tumor: increase in abundance of CD4+, CD8+, naïve T, B and NK T cells • In tumor microenvironment: increase in CD8 T, CD4 T, naïve and B cells • Over 200 immune cell genes activated
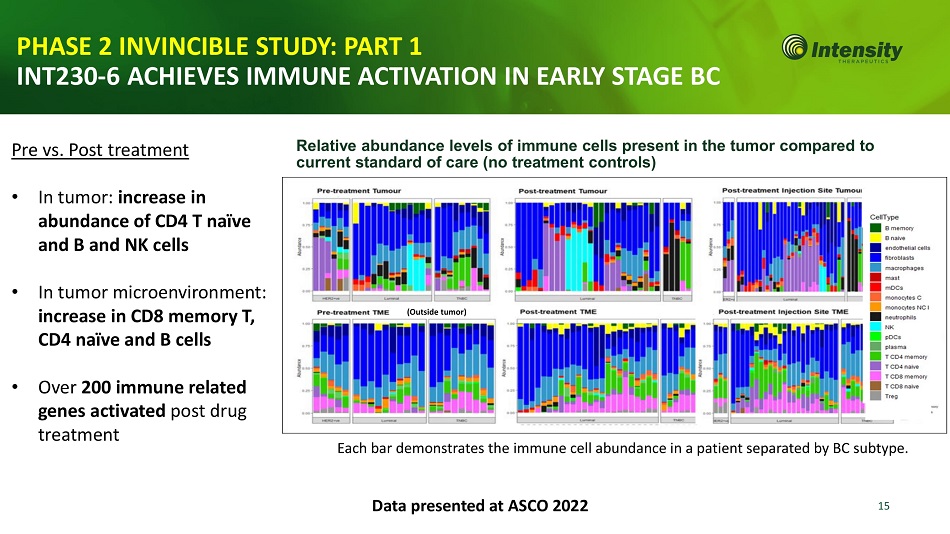
15 PHASE 2 INVINCIBLE STUDY: PART 1 INT230 - 6 ACHIEVES IMMUNE ACTIVATION IN EARLY STAGE BC Relative abundance levels of immune cells present in the tumor compared to current standard of care (no treatment controls) Each bar demonstrates the immune cell abundance in a patient separated by BC subtype. Data presented at ASCO 2022 (Outside tumor) Pre vs. Post treatment • In tumor: increase in abundance of CD4 T naïve and B and NK cells • In tumor microenvironment: increase in CD8 memory T, CD4 naïve and B cells • Over 200 immune related genes activated post drug treatment

16 INVINCIBLE STUDY SAFETY, EFFICACY AND NEXT DATA SETS PART 2 (ongoing analysis): INT230 - 6 treatment compared to saline sham injection Major pathological response (MPR) defined as < 10% residual cancer in tumor MPR was 6% overall in study with ~14.3% in tumors >2.9 cm compared to control (0%) p=0.042 Evaluate other immunomodulatory (T - cell repertoire) and biologic effects Potential Design of Phase 3 Program: INT230 - 6 + Standard of care in Triple Negative Breast Cancer and/or HER2+

17 DATA PRESENTED AT ASCO 2022 Results in Metastatic Cancers: INT230 - 6 Monotherapy or with immunotherapy
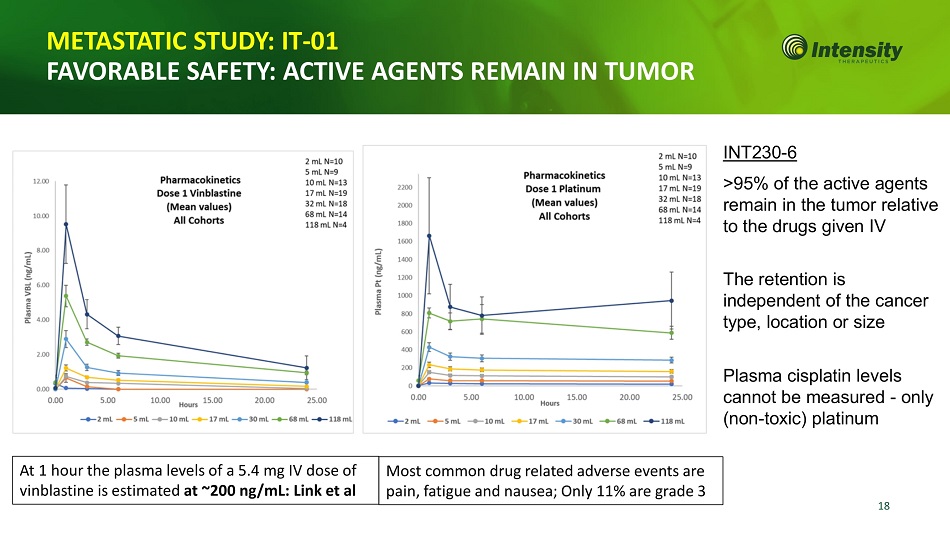
18 METASTATIC STUDY: IT - 01 FAVORABLE SAFETY: ACTIVE AGENTS REMAIN IN TUMOR INT230 - 6 >95% of the active agents remain in the tumor relative to the drugs given IV The retention is independent of the cancer type, location or size Plasma cisplatin levels cannot be measured - only (non - toxic) platinum At 1 hour the plasma levels of a 5.4 mg IV dose of vinblastine is estimated at ~200 ng/mL: Link et al Most common drug related adverse events are pain, fatigue and nausea; Only 11% are grade 3
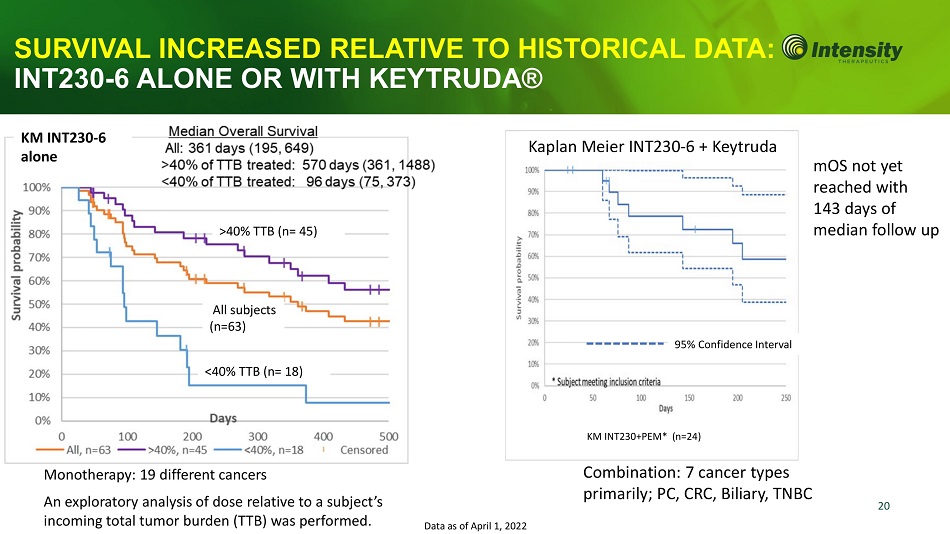
20 All subjects (n=63) >40% TTB (n= 45) <40% TTB (n= 18) KM INT230+PEM* (n=24) KM INT230 - 6 alone mOS not yet reached with 143 days of median follow up Monotherapy: 19 different cancers An exploratory analysis of dose relative to a subject’s incoming total tumor burden (TTB) was performed. Data as of April 1, 2022 SURVIVAL INCREASED RELATIVE TO HISTORICAL DAT INT230 - 6 ALONE OR WITH KEYTRUDA® Kaplan Meier INT230 - 6 + Keytruda Combination: 7 cancer types primarily; PC, CRC, Biliary, TNBC 95% Confidence Interval
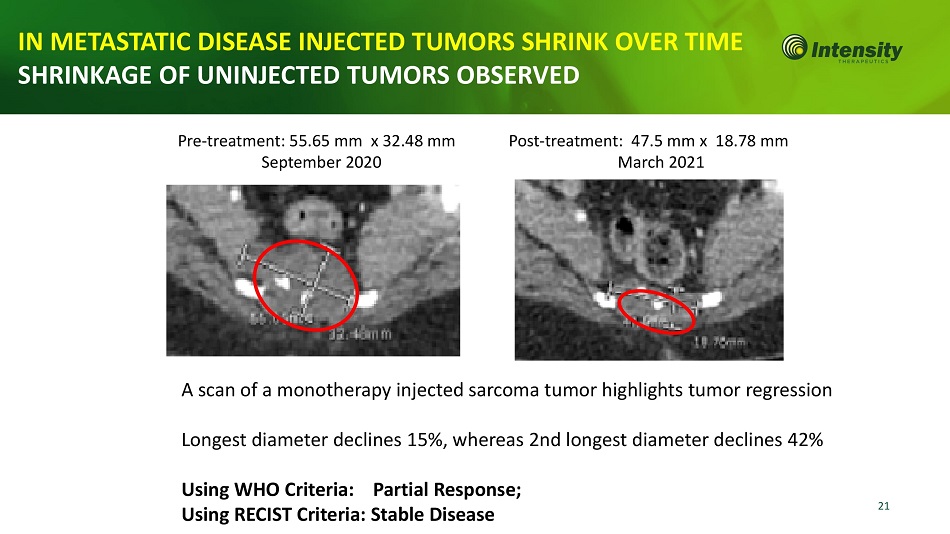
21 IN METASTATIC DISEASE INJECTED TUMORS SHRINK OVER TIME SHRINKAGE OF UNINJECTED TUMORS OBSERVED A scan of a monotherapy injected sarcoma tumor highlights tumor regression Longest diameter declines 15%, whereas 2nd longest diameter declines 42% Using WHO Criteria: Partial Response; Using RECIST Criteria: Stable Disease Pre - treatment: 55.65 mm x 32.48 mm September 2020 Post - treatment: 47.5 mm x 18.78 mm March 2021
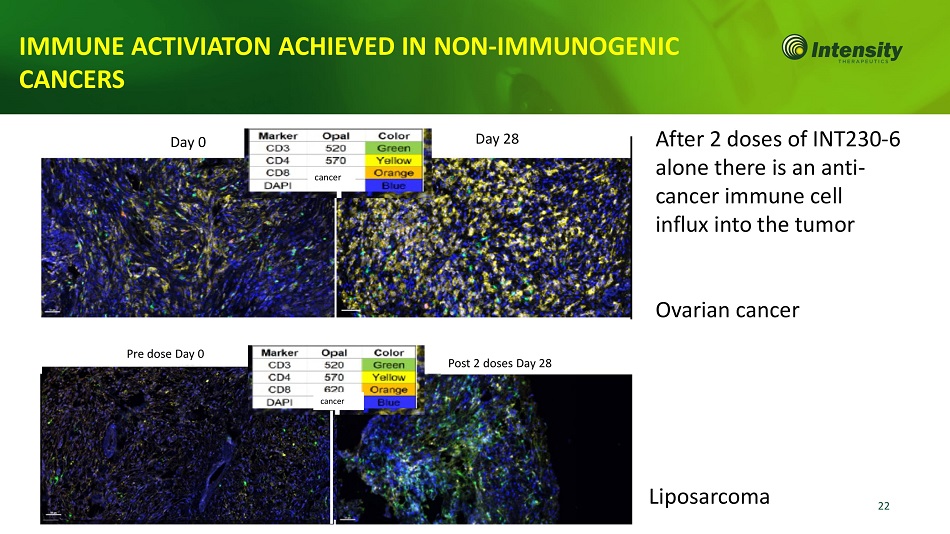
22 Post 2 doses Day 28 Pre dose Day 0 Liposarcoma cancer IMMUNE ACTIVIATON ACHIEVED IN NON - IMMUNOGENIC CANCERS After 2 doses of INT230 - 6 alone there is an anti - cancer immune cell influx into the tumor Ovarian cancer Day 0 Day 28 cancer
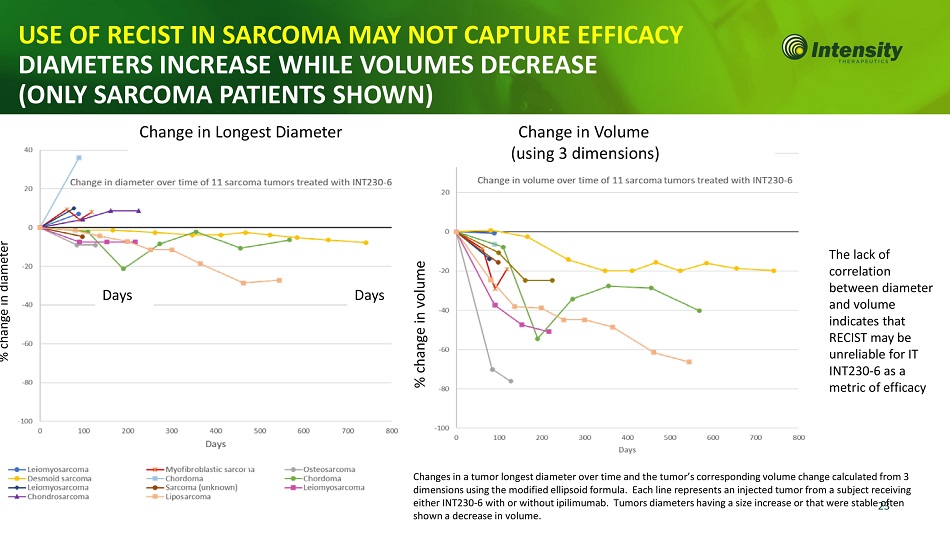
Change in Longest Diameter Change in Volume (using 3 dimensions) The lack of correlation between diameter and volume indicates that RECIST may be unreliable for IT INT230 - 6 as a metric of efficacy Days % change in diameter % change in volume Days Changes in a tumor longest diameter over time and the tumor’s corresponding volume change calculated from 3 dimensions using the modified ellipsoid formula. Each line represents an injected tumor from a subject receiving either INT230 - 6 with or without ipilimumab. Tumors diameters having a size increase or that were stable 2 o 3 ften shown a decrease in volume. USE OF RECIST IN SARCOMA MAY NOT CAPTURE EFFICACY DIAMETERS INCREASE WHILE VOLUMES DECREASE (ONLY SARCOMA PATIENTS SHOWN)
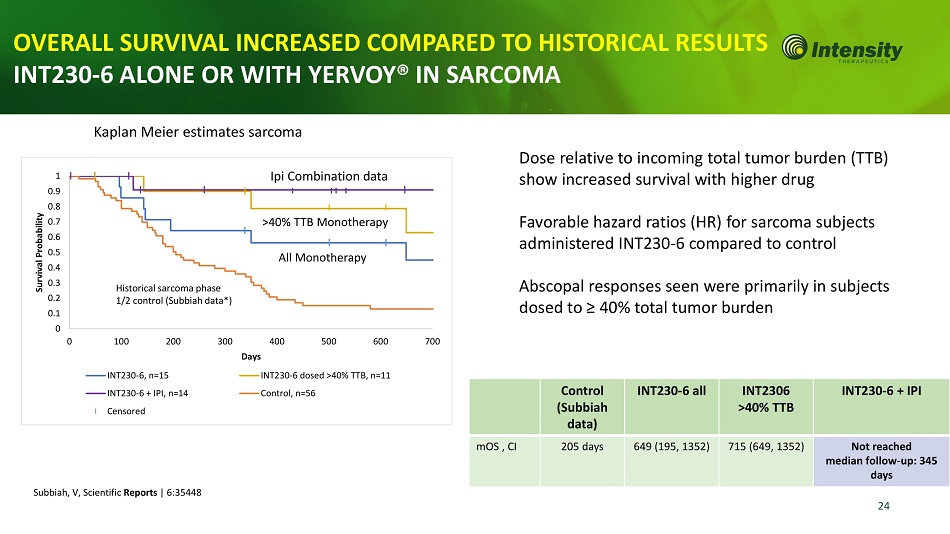
24 1 0 .9 0 .8 0 .7 0 .6 0 .5 0 .4 Survival Probability INT230 - 6 dosed >40% TTB, n=11 Control, n=56 INT230 - 6, n=15 INT230 - 6 + IPI, n=14 Censored 0.3 Historical sarcoma phase 0.2 1/2 control (Subbiah data*) 0.1 0 0 100 200 300 400 500 600 700 Days Kaplan Meier estimates sarcoma Ipi Combination data Control ( S ubbiah data) INT230 - 6 all INT2306 >40% TTB INT230 - 6 + IPI mOS , CI 205 days 649 (195, 1352) 715 (649, 1352) Not reached median follow - up: 345 days Dose relative to incoming total tumor burden (TTB) show increased survival with higher drug Favorable hazard ratios (HR) for sarcoma subjects administered INT230 - 6 compared to control Abscopal responses seen were primarily in subjects dosed to ≥ 40% total tumor burden OVERALL SURVIVAL INCREASED COMPARED TO HISTORICAL RESULT INT230 - 6 ALONE OR WITH YERVOY® IN SARCOMA Subbiah, V, Scientific Reports | 6:35448 >40% TTB Monotherapy All Monotherapy
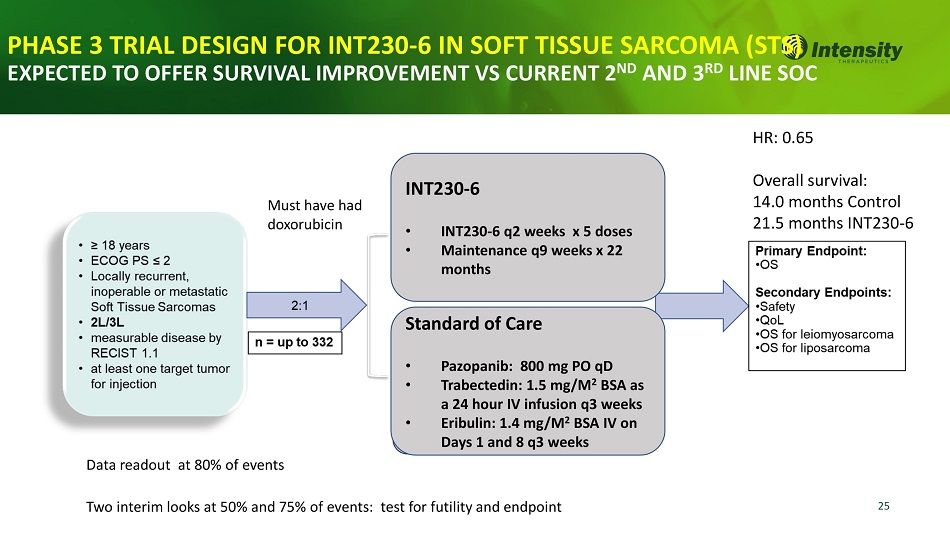
25 Two interim looks at 50% and 75% of events: test for futility and endpoint Must have had doxorubicin HR: 0.65 Overall survival: 14.0 months Control 21.5 months INT230 - 6 Data readout at 80% of events PHASE 3 TRIAL DESIGN FOR INT230 - 6 IN SOFT TISSUE SARCOMA S) EXPECTED TO OFFER SURVIVAL IMPROVEMENT VS CURRENT 2 ND AND 3 RD LINE SOC INT230 - 6 • INT230 - 6 q2 weeks x 5 doses • Maintenance q9 weeks x 22 months Standard of Care • Pazopanib: 800 mg PO qD • Trabectedin: 1.5 mg/M 2 BSA as a 24 hour IV infusion q3 weeks • Eribulin: 1.4 mg/M 2 BSA IV on Days 1 and 8 q3 weeks

26 • INT230 - 6 has induced significant necrosis in large tumors following a single dose • Immune activation observed of non - immunogenic cancer types: potential for presurgical use • Favorable safety and promising increased survival efficacy • INT230 - 6 represents a new approach to cancer treatment (immunological cell killing) • Interest from: academic hospitals, major clinical oncology societies, big pharma, government (NCI, OICR) • Planned phase 3 programs - important market opportunities: • Breast cancer: pre - surgery - with chemo 30,000 patients; no chemo: 170,000 cases; (US) • Metastatic sarcoma: 157,000 patients in US; 12,000 new cases each year; (US) CONCLUSIONS AND FIRST MARKET OPPORTUNITIES
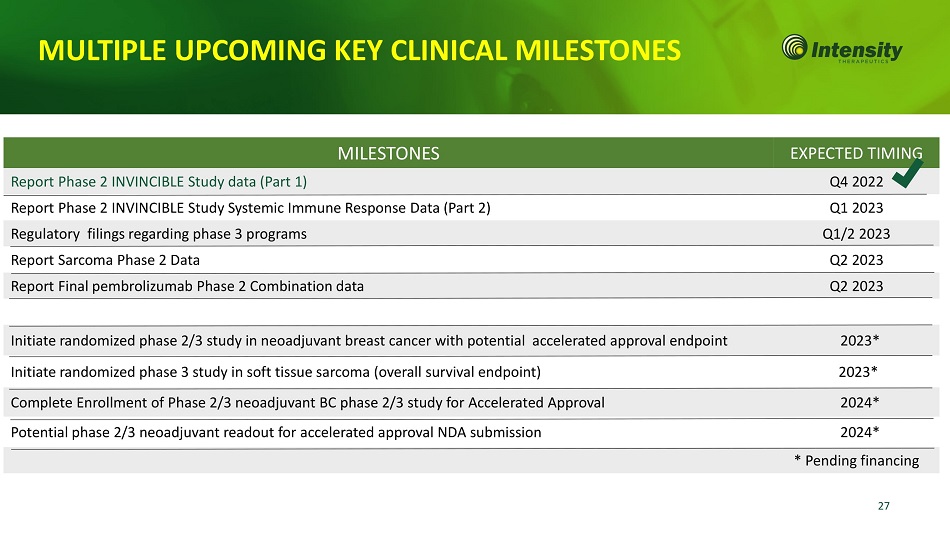
27 MULTIPLE UPCOMING KEY CLINICAL MILESTONES MILESTONES EXPECTED TIMING Report Phase 2 INVINCIBLE Study data (Part 1) Q4 2022 Report Phase 2 INVINCIBLE Study Systemic Immune Response Data (Part 2) Q1 2023 Regulatory filings regarding phase 3 programs Q1/2 2023 Report Sarcoma Phase 2 Data Q2 2023 Report Final pembrolizumab Phase 2 Combination data Q2 2023 Initiate randomized phase 2/3 study in neoadjuvant breast cancer with potential accelerated approval endpoint 2023* Initiate randomized phase 3 study in soft tissue sarcoma (overall survival endpoint) 2023* Complete Enrollment of Phase 2/3 neoadjuvant BC phase 2/3 study for Accelerated Approval 2024* Potential phase 2/3 neoadjuvant readout for accelerated approval NDA submission 2024* * Pending financing

28 INTENSITY THERAPEUTICS A NEW WEAPON IN THE WAR ON CANCER Contact Investor Relations Contact : Rx Communications Group Michael Miller (917) - 633 - 6086 mmiller@rxir.com Thank you!