
Exhibit 99.2

A New Weapon in the War on Cancer September 2023 Issuer Free Writing Prospectus Filed pursuant to Rule 433 Registration Number 333 - 260565

Highlights • Novel cancer treatment approach with first - in - class compound that causes cancer cell death leading to an immune response for indications with high unmet medical need • Late - stage pipeline programs in metastatic and presurgical settings with multiple near - term inflexion points • Experienced leadership team from Emisphere, Roche and Bristol Myers; CEO has public company as well as biopharma development and commercial experience • Robust IP portfolio, platform validated through multiple Industry, government and university hospital partnerships • De - risked and cost - efficient business model structured to create significant value 2

Platform Validated by World Leading Partners RESEARCH CLINICAL TRIAL SITES 3

4 Management Team: Extensive Oncology and Drug Development Experience Veteran Operators with Public Company and IPO Experience • Drug delivery expertise Preclinical through Phase 3 • Public biotech company CEO experience Daniel Donovan CEO Rare Life Emer Leahy, Ph.D. CEO Psychogenics Mark A. Goldberg, MD Former President & COO of PAREXEL Lewis H. Bender CEO Intensity Lewis H. Bender, MIT ChE, MS, MA, MBA Founder, CEO John Wesolowski, MBA, CPA Chief Financial Officer, Principal Accounting Officer and Controller Brian Schwartz, MD Clinical Development CE O , CT O , V P, B D & Manufacturing Manufacturing CEO James M. Ahlers Executive Vice President – Corporate Finance • 25 years, multiple transactions • Titan Pharmaceutics, IPO BOARD OF DIRECTORS KEY MANAGEMENT Ian Walters Medical Advisor Steve Innaimo Project Management Rebecca Drain, Doranne Frano Regulatory & Quality Rita Cooney PH.D. Analytical Chemistry Karen Du Clinical Operations Joseph Bernadino, George Klein Manufacturing
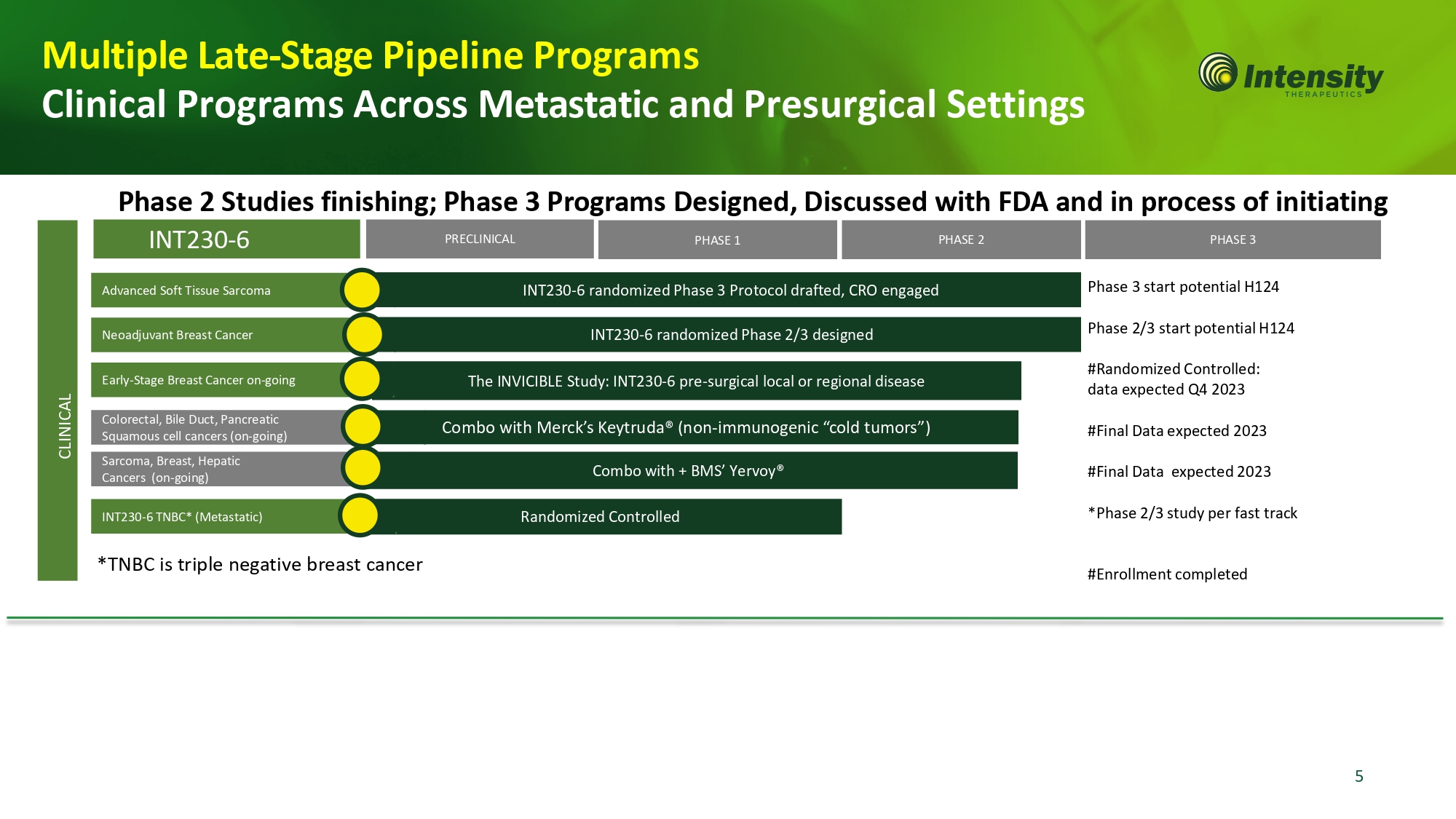
Advanced Soft Tissue Sarcoma INT230 - 6 randomized Phase 3 Protocol drafted, CRO engaged PHASE 3 PHASE 2 PHASE 1 PRECLINICAL INT230 - 6 Colorectal, Bile Duct, Pancreatic Squamous cell cancers (on - going) Sarcoma, Breast, Hepatic Cancers (on - going) Combo with + BMS’ Yervoy® Combo with Merck’s Keytruda® (non - immunogenic “cold tumors”) Multiple Late - Stage Pipeline Programs Clinical Programs Across Metastatic and Presurgical Settings Phase 2 Studies finishing; Phase 3 Programs Designed, Discussed with FDA and in process of initiating The INVICIBLE Study: INT230 - 6 pre - surgical local or regional disease Early - Stage Breast Cancer on - going CLI N IC AL P ha se 3 st ar t p o te n t ia l H 124 Phase 2/3 start potential H124 #R and om iz ed C o n t r o ll e d : data expected Q4 2023 #Final Data expected 2023 #Final Data expected 2023 *Phase 2/3 study per fast track #E nr o ll m e n t c om pl e t ed *TNBC is triple negative breast cancer R and om iz ed C o n t r o ll ed I N T 230 - 6 T N BC* ( Met a st a ti c) Neoadjuvant Breast Cancer INT230 - 6 randomized Phase 2/3 designed 5

• Phase 3 programs - important market opportunities: Metastatic sarcoma : • 157,000 patients in US; • 12,000 new cases per year (6,000 deaths); (US) • Estimated annual revenue per patient based on phase 2 use Breast Cancer • ~287,850 new cases of invasive breast cancer diagnosed in women in the U.S. during 2022 • About 1 in 8 U.S. women (about 13%) will develop invasive breast cancer over the course of her lifetime Presurgical breast cancer : • INT230 - 6 with Standard of Care (SOC) chemotherapy: 30,000 patients US • w/out chemotherapy: 60,000 Large tumor cases; (US) INT230 - 6 vs. no treatment (current SOC) 6 First Two Clinical Market Opportunities
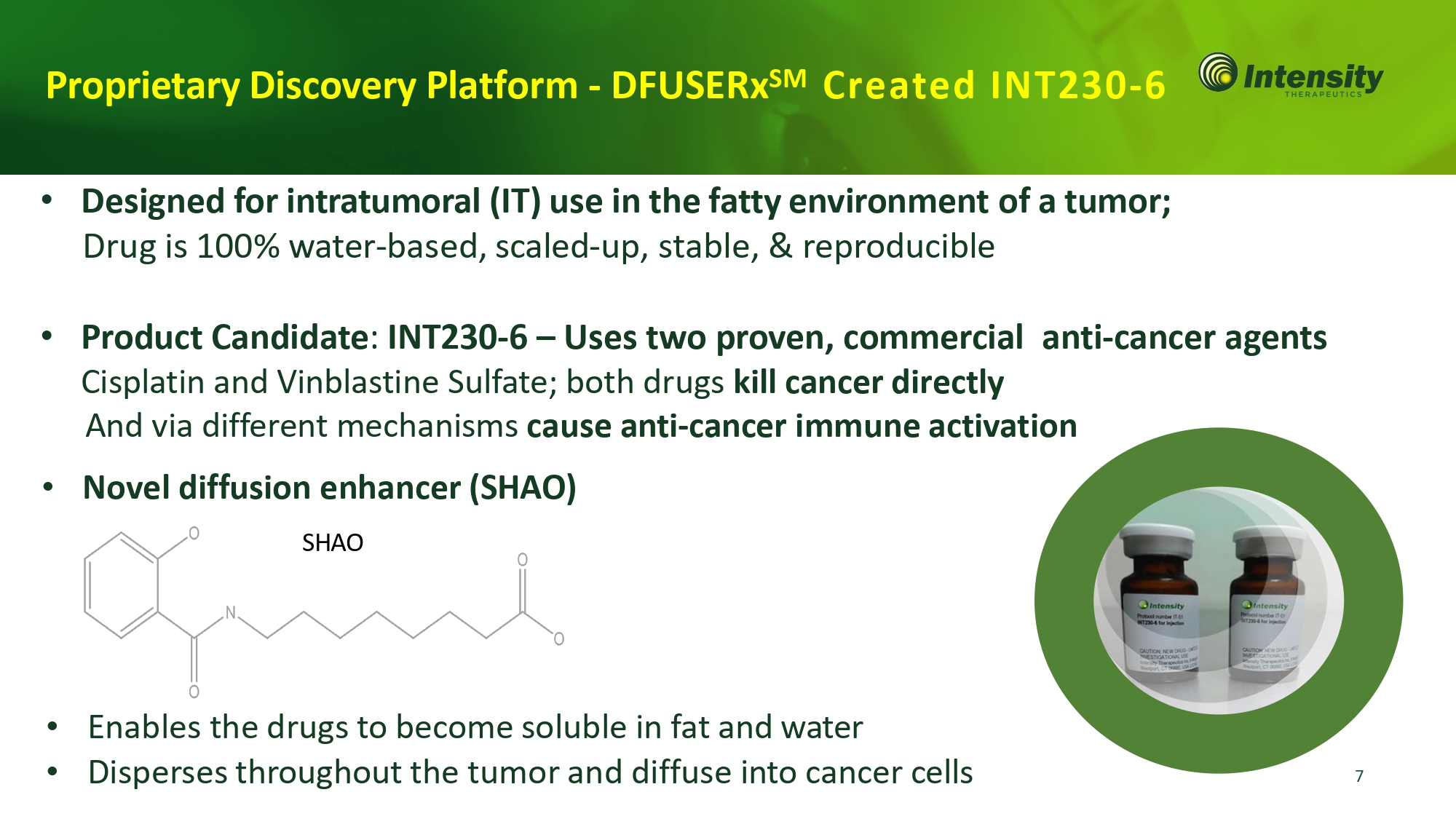
7 Proprietary Discovery Platform - DFUSERx SM Created INT230 - • Designed for intratumoral (IT) use in the fatty environment of a tumor; Drug is 100% water - based, scaled - up, stable, & reproducible • Product Candidate : INT230 - 6 – Uses two proven, commercial anti - cancer agents Cisplatin and Vinblastine Sulfate; both drugs kill cancer directly And via different mechanisms cause anti - cancer immune activation • Novel diffusion enhancer (SHAO) SHAO • Enables the drugs to become soluble in fat and water • Disperses throughout the tumor and diffuse into cancer cells
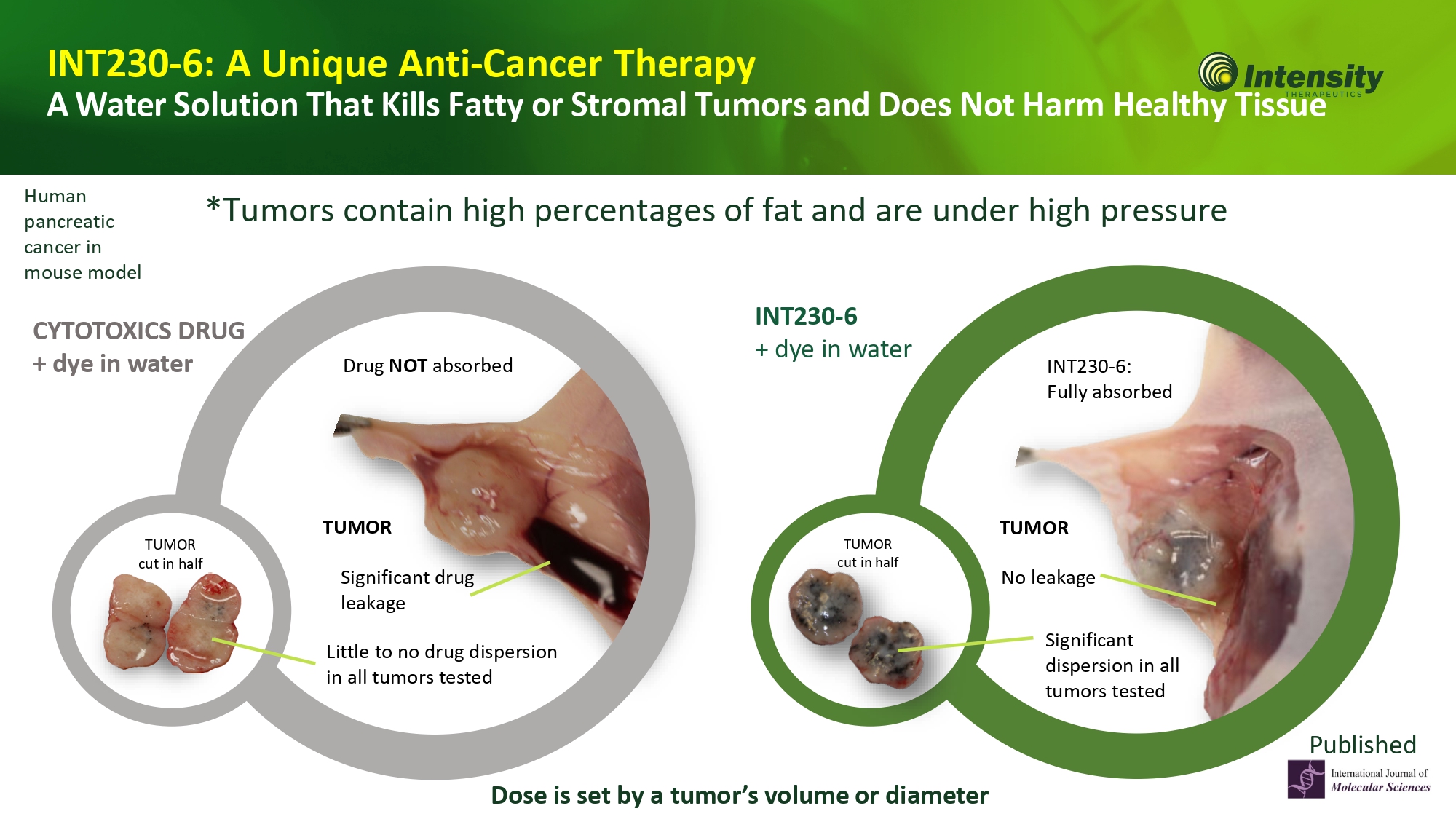
10 INT230 - 6: A Unique Anti - Cancer Therapy A Water Solution That Kills Fatty or Stromal Tumors and Does Not Harm Healthy Tissue *Tumors contain high percentages of fat and are under high pressure CYTOTOXICS DRUG + dye in water Little to no drug dispersion in all tumors tested TUMOR cut in half Significant drug leakage Drug NOT absorbed Human pancreatic cancer in mouse model TUMOR cut in half Significant dispersion in all tumors tested No leakage INT230 - 6: Fully absorbed INT230 - 6 + dye in water Dose is set by a tumor’s volume or diameter T UMO R T UMO R Published

Patients Enrolled: 91 – Complete INT230 - 6 R andomized to either no treatment or saline injection • Site: Ottawa Hospital • Investigator: Dr. Angel Arnaout • Objectives : Cause sufficient tumor necrosis prior to surgery to activate the immune system and determine INT230 - 6 safety presurgically • Final Goal: Reduce the risk of disease recurrence Our clinical results have been selected for Spotlight Oral Podium Presentation at: The San Antonio Breast Cancer Symposium (SABCS) annual meeting December 2022: 11 Design: Randomized, Placebo Controlled Phase 2 Window Trial In Presurgical Breast Cancer (BC): The INVINCIBLE Study
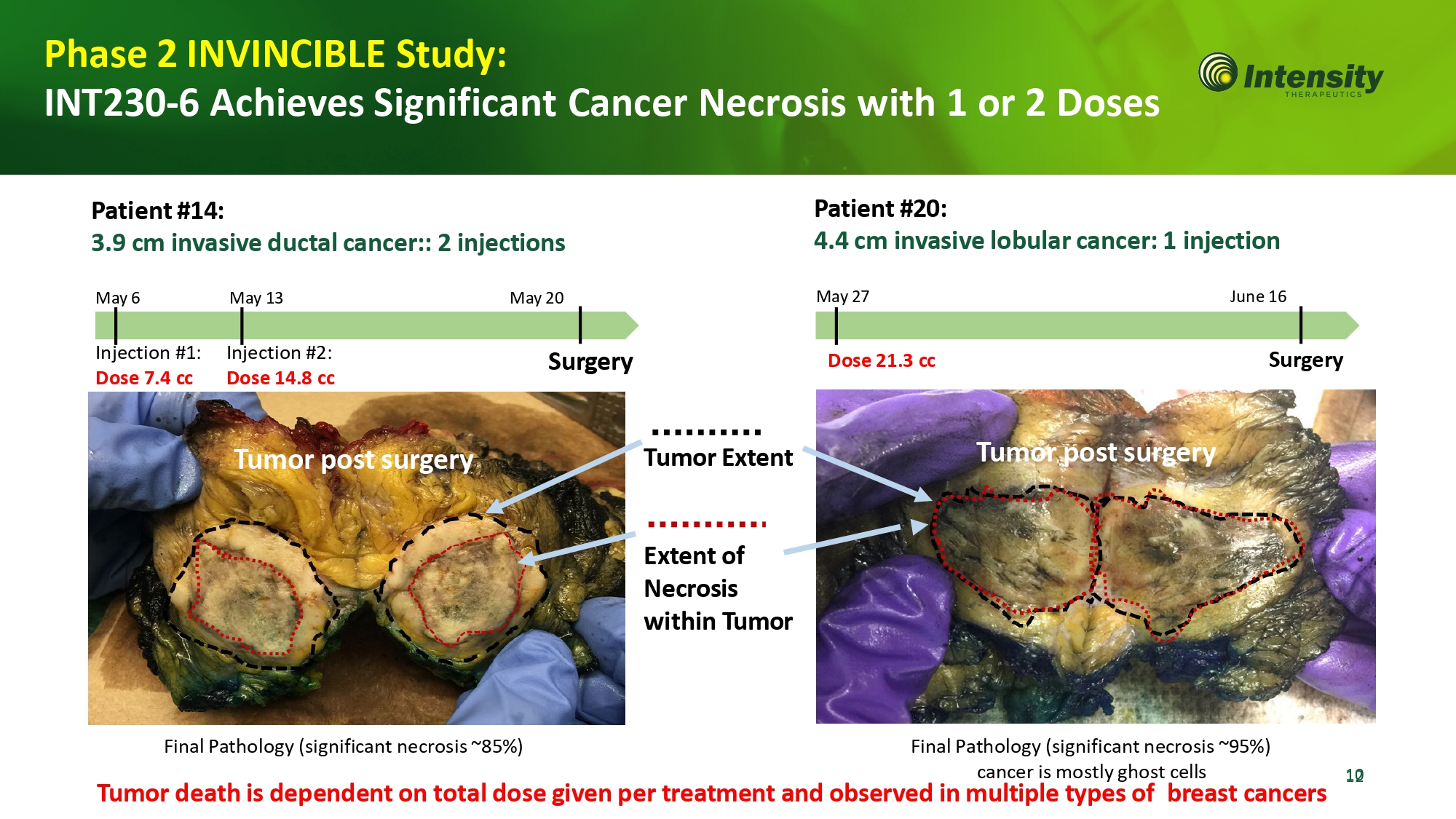
1 0 2 T u m o r Ex t e n t E x t e n t o f Necrosis wit h in T u m o r Phase 2 INVINCIBLE Study: INT230 - 6 Achieves Significant Cancer Necrosis with 1 or 2 Doses P a ti e n t #14 : 3.9 cm invasive ductal cancer:: 2 injections M a y 6 M a y 1 3 M a y 20 Final Pathology (significant necrosis ~85%) Final Pathology (significant necrosis ~95%) cancer is mostly ghost cells Tumor death is dependent on total dose given per treatment and observed in multiple types of breast cancers In j e c t ion #1: Dose 7.4 cc Injection #2: Dose 14. 8 c c S u rg e r y P a ti e n t #20 : 4.4 cm invasive lobular cancer: 1 injection M a y 2 7 J un e 16 Dose 21. 3 c c S u r g e r y T u mo r po s t su rg e r y T u mo r po s t su rg e r y
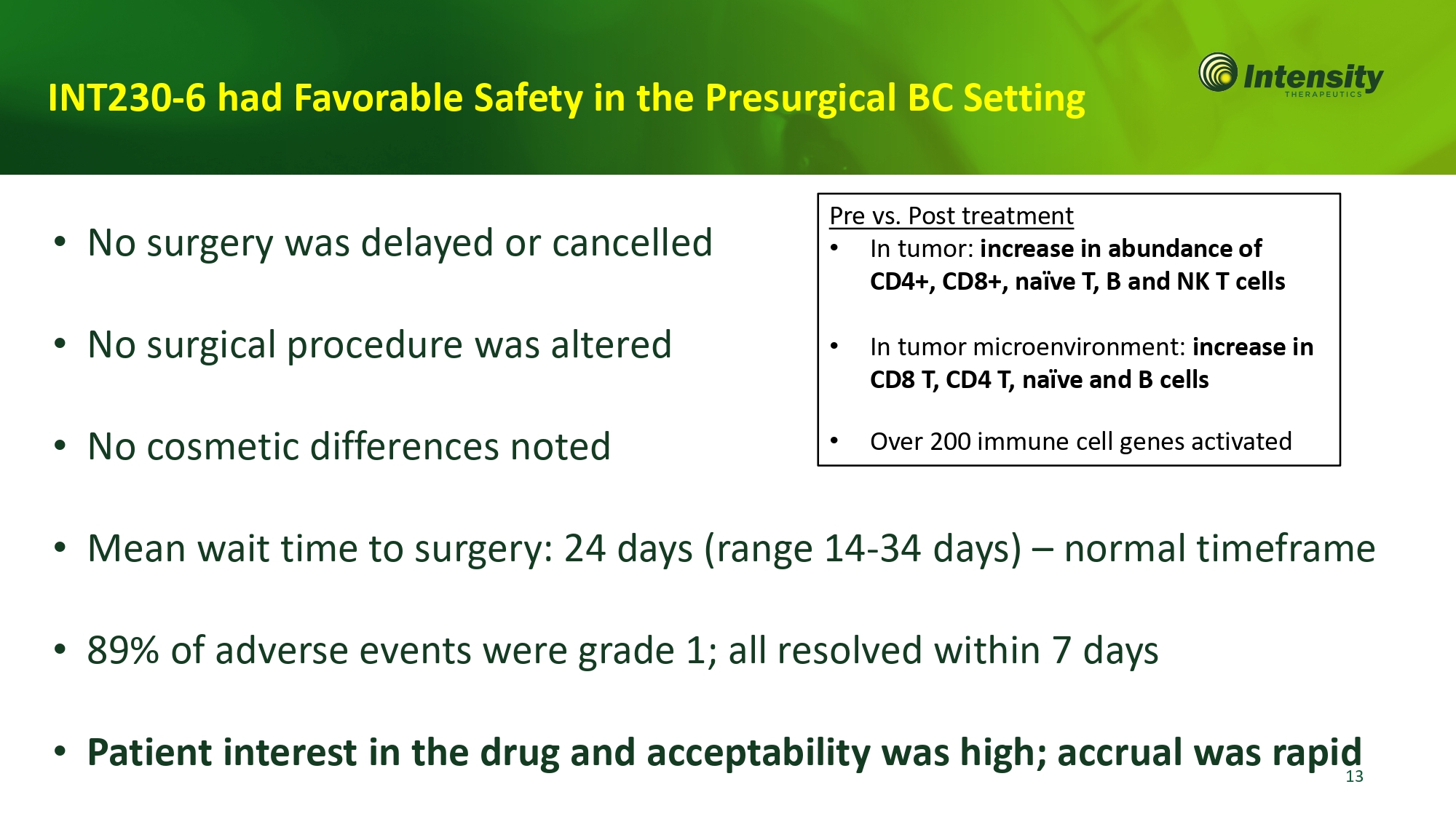
INT230 - 6 had Favorable Safety in the Presurgical BC Setting • No surgery was delayed or cancelled • No surgical procedure was altered • No cosmetic differences noted • Mean wait time to surgery: 24 days (range 14 - 34 days) – normal timeframe • 89% of adverse events were grade 1; all resolved within 7 days • Patient interest in the drug and acceptability was high; accrual was rapid Pre vs. Post treatment • In tumor: increase in abundance of CD4+, CD8+, naïve T, B and NK T cells • In tumor microenvironment: increase in CD8 T, CD4 T, naïve and B cells • Over 200 immune cell genes activated 13
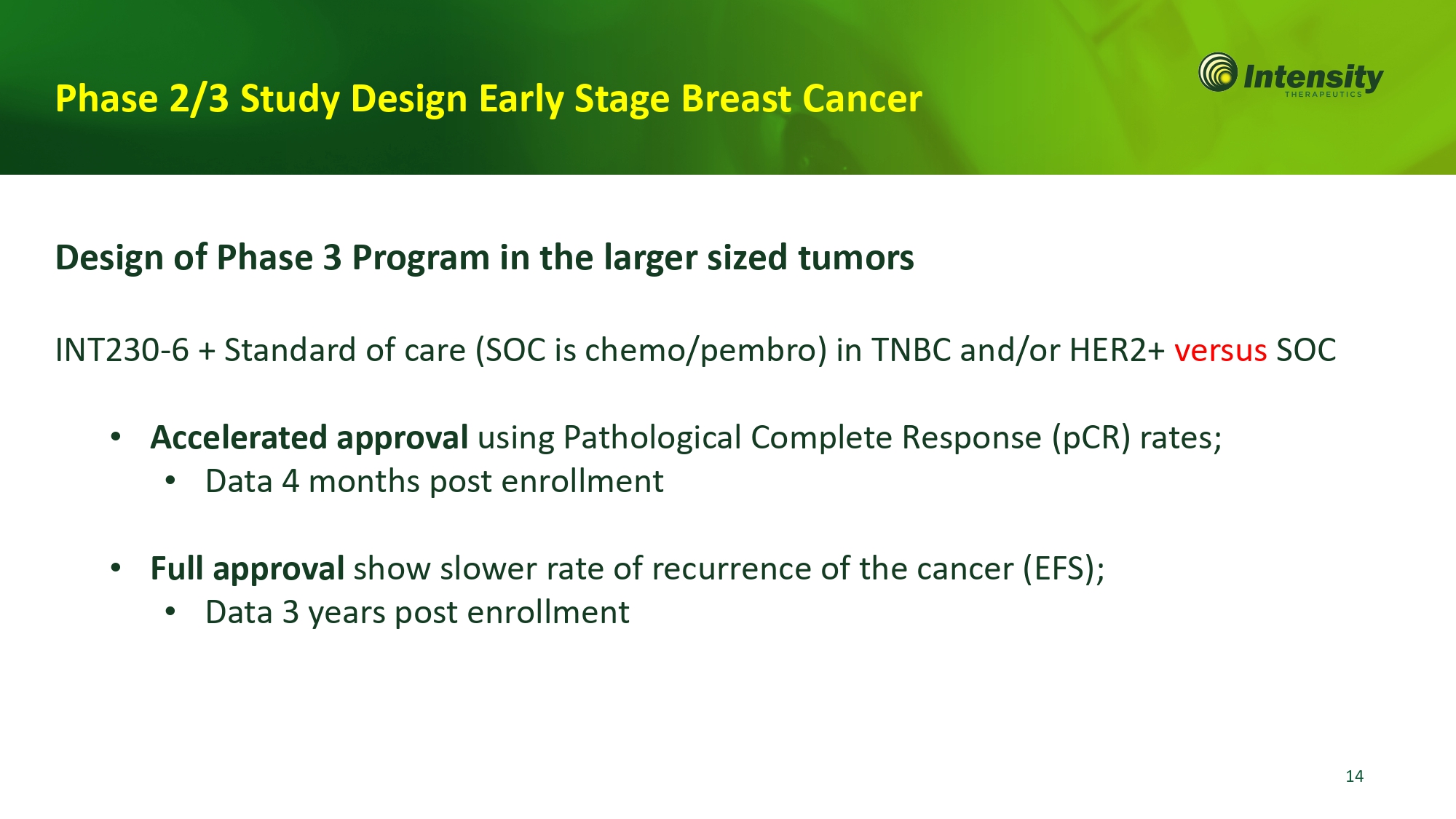
Phase 2/3 Study Design Early Stage Breast Cancer 14 Design of Phase 3 Program in the larger sized tumors INT230 - 6 + Standard of care (SOC is chemo/pembro) in TNBC and/or HER2+ versus SOC • Accelerated approval using Pathological Complete Response (pCR) rates; • Data 4 months post enrollment • Full approval show slower rate of recurrence of the cancer (EFS); • Data 3 years post enrollment
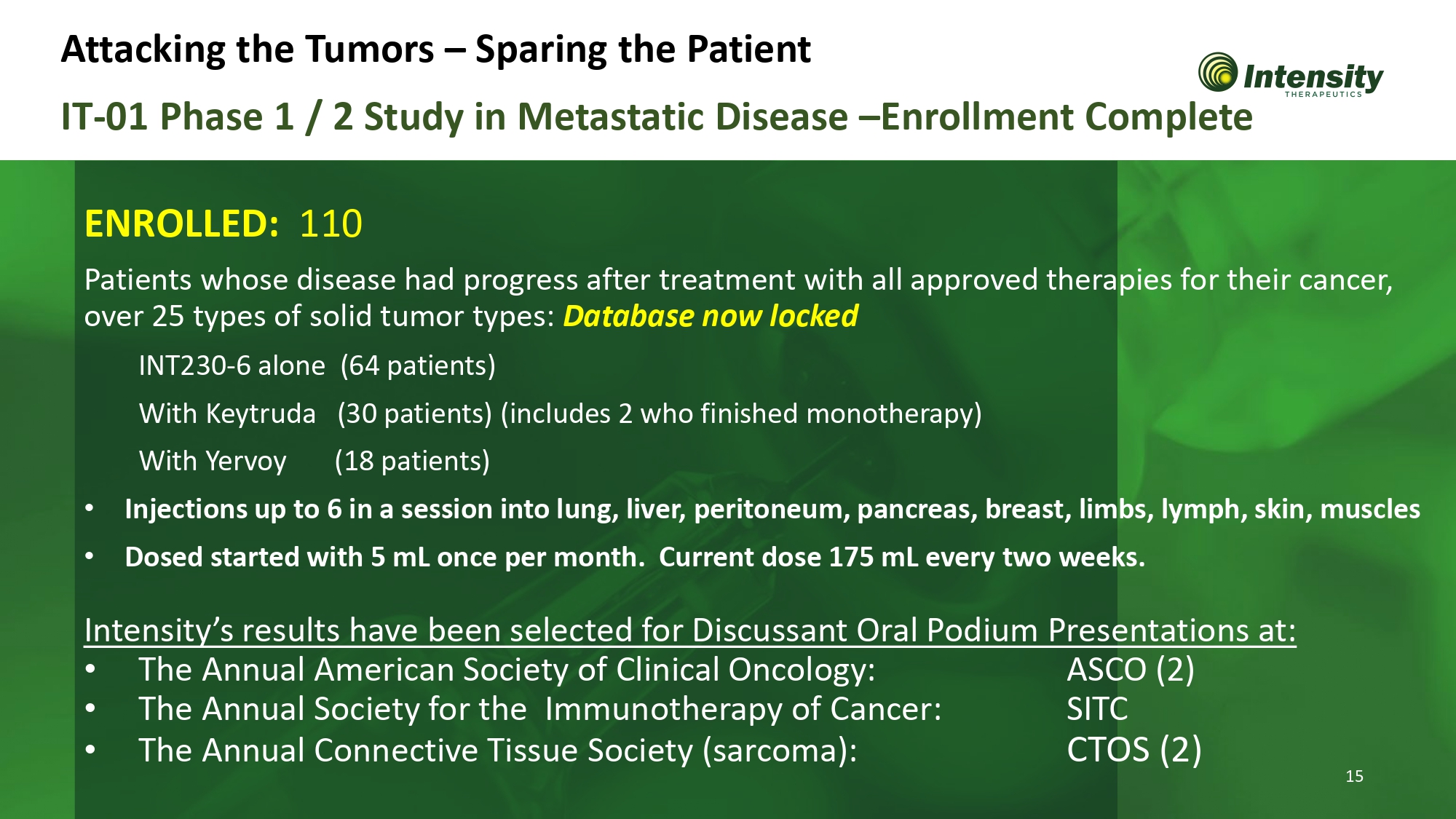
IT - 01 Phase 1 / 2 Study in Metastatic Disease – Enrollment Complete ENROLLED: 110 15 Patients whose disease had progress after treatment with all approved therapies for their cancer, over 25 types of solid tumor types: Database now locked INT230 - 6 alone (64 patients) With Keytruda (30 patients) (includes 2 who finished monotherapy) With Yervoy (18 patients) • Injections up to 6 in a session into lung, liver, peritoneum, pancreas, breast, limbs, lymph, skin, muscles • Dosed started with 5 mL once per month. Current dose 175 mL every two weeks. Intensity’s results have been selected for Discussant Oral Podium Presentations at: ASCO (2) S I TC • The Annual American Society of Clinical Oncology: • The Annual Society for the Immunotherapy of Cancer: • The Annual Connective Tissue Society (sarcoma): CTOS (2) Attacking the Tumors – Sparing the Patient

Favorable Safety: Active Agents Remain in the Tumor 17 INT230 - 6 >95% of the active agents remain in the tumor relative to the drugs given IV The retention is independent of the cancer type, location or size Most common drug related adverse events are mild or moderate injection site pain, fatigue and brief nausea ~90 are low grade; No grade 4 or grade 5 related adverse events.
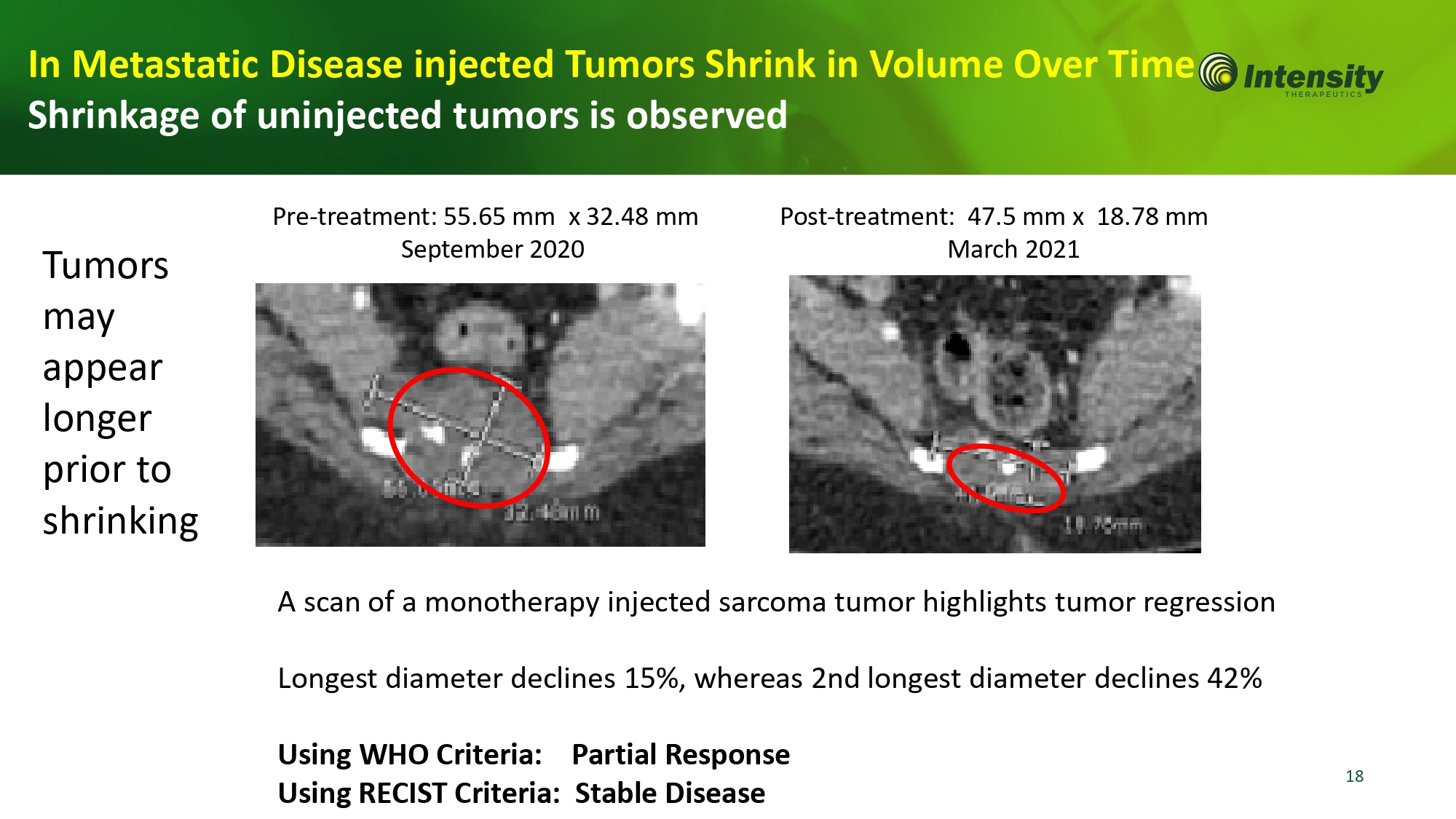
18 In Metastatic Disease injected Tumors Shrink in Volume Over Tim Shrinkage of uninjected tumors is observed A scan of a monotherapy injected sarcoma tumor highlights tumor regression Longest diameter declines 15%, whereas 2nd longest diameter declines 42% Using WHO Criteria: Partial Response Using RECIST Criteria: Stable Disease Pre - treatment: 55.65 mm x 32.48 mm September 2020 Post - treatment: 47.5 mm x 18.78 mm March 2021 Tumors may appear longer prior to shr in k in g
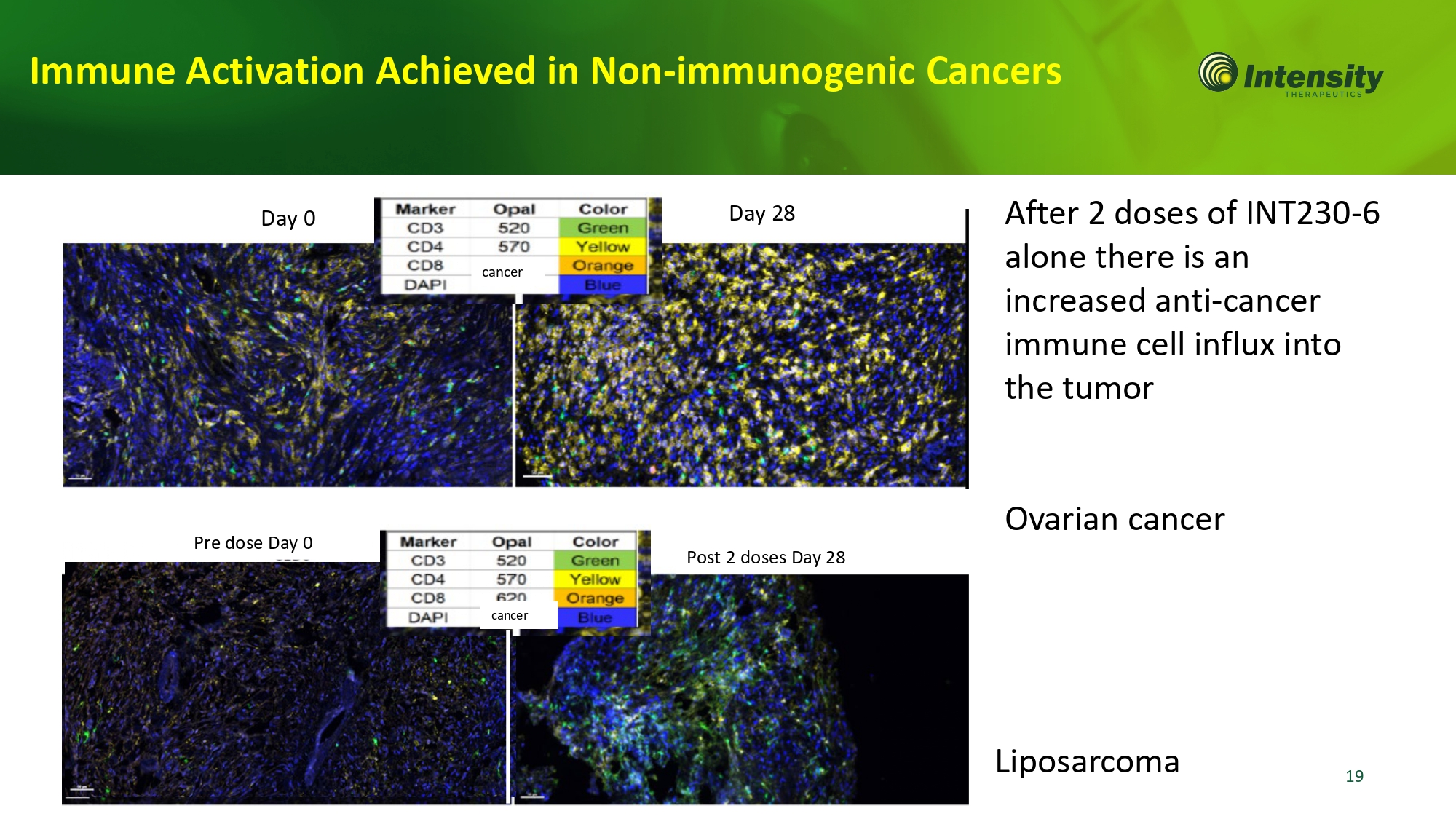
19 Post 2 doses Day 28 Pre dose Day 0 Liposarcoma cancer Immune Activation Achieved in Non - immunogenic Cancers After 2 doses of INT230 - 6 alone there is an increased anti - cancer immune cell influx into the tumor Ovarian cancer Day 0 Day 28 cancer
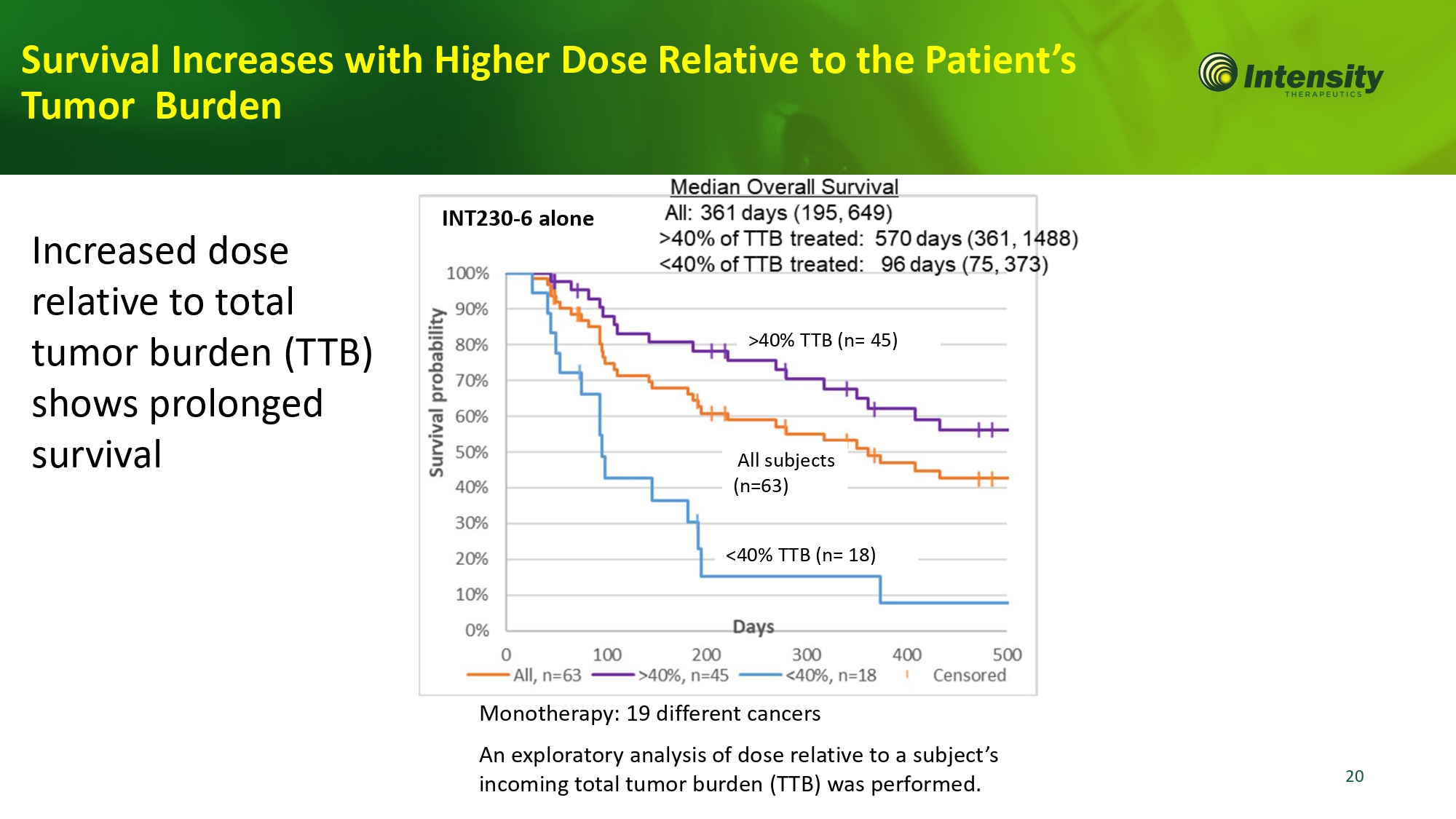
20 All subjects (n=63) >40% TTB (n= 45) <40% TTB (n= 18) INT230 - 6 alone Monotherapy: 19 different cancers An exploratory analysis of dose relative to a subject’s incoming total tumor burden (TTB) was performed. Survival Increases with Higher Dose Relative to the Patient’s Tumor Burden Increased dose relative to total tumor burden (TTB) shows prolonged survival
Sarcoma: A Deadly and Painful Cancer 21
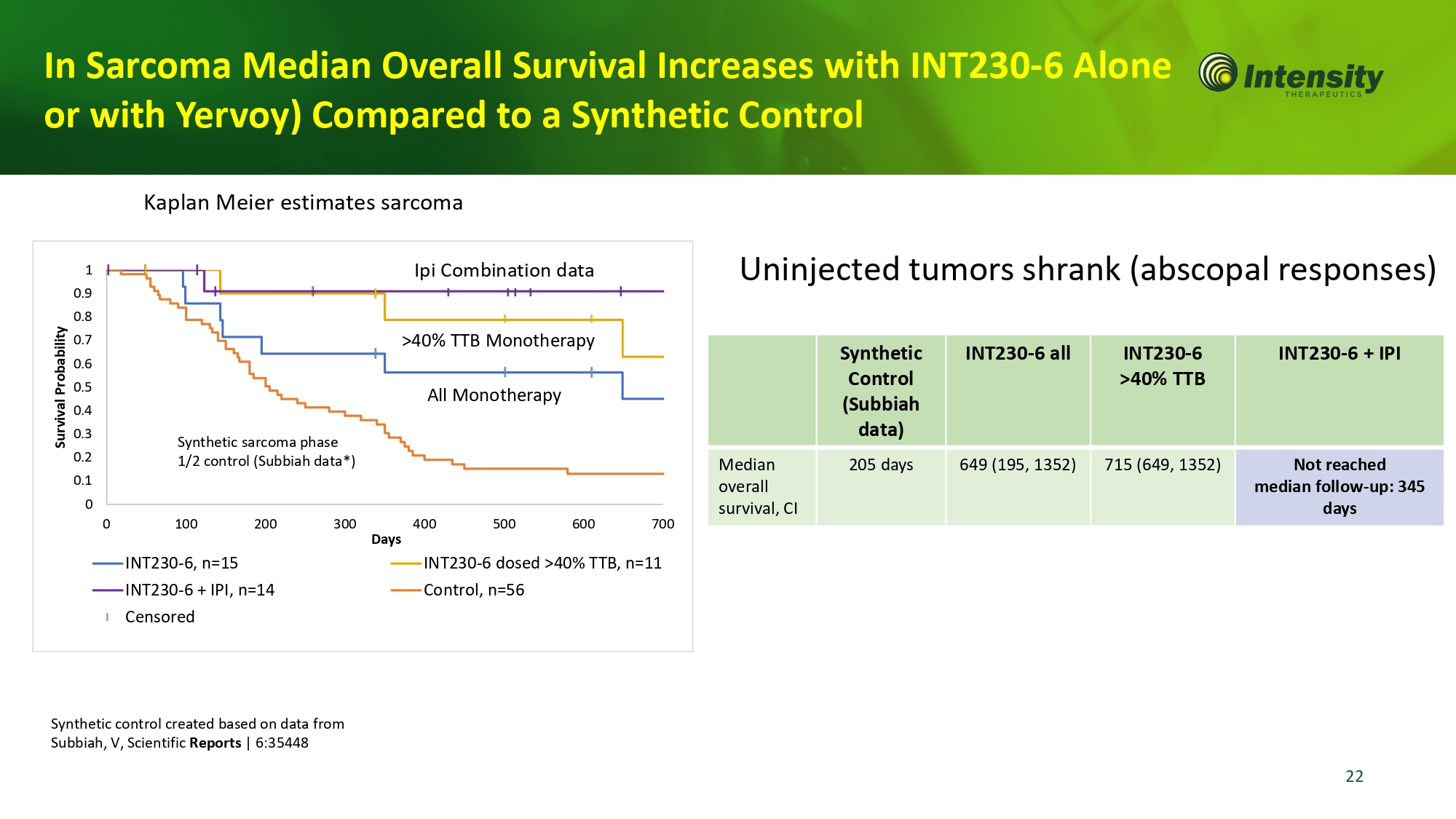
1 0 .9 0 .8 0 .7 0 .6 0 .5 0 .4 Survival Probability 0.3 Synthetic sarcoma phase 0.2 1/2 control (Subbiah data*) 0.1 0 0 100 200 300 400 500 600 700 Days INT230 - 6, n=15 INT230 - 6 dosed >40% TTB, n=11 INT230 - 6 + IPI, n=14 Control, n=56 Censored Kaplan Meier estimates sarcoma Ipi Combination data INT230 - 6 + IPI INT230 - 6 >40% TTB INT230 - 6 all S y n t h e t ic Control (Subbiah data) Not reached median follow - up: 345 days 715 (649, 1352) 649 (195, 1352) 205 days Median overall s u r v i v al, C I Uninjected tumors shrank (abscopal responses) In Sarcoma Median Overall Survival Increases with INT230 - 6 Alon or with Yervoy) Compared to a Synthetic Control Synthetic control created based on data from Subbiah, V, Scientific Reports | 6:35448 >40% TTB Monotherapy All Monotherapy 22
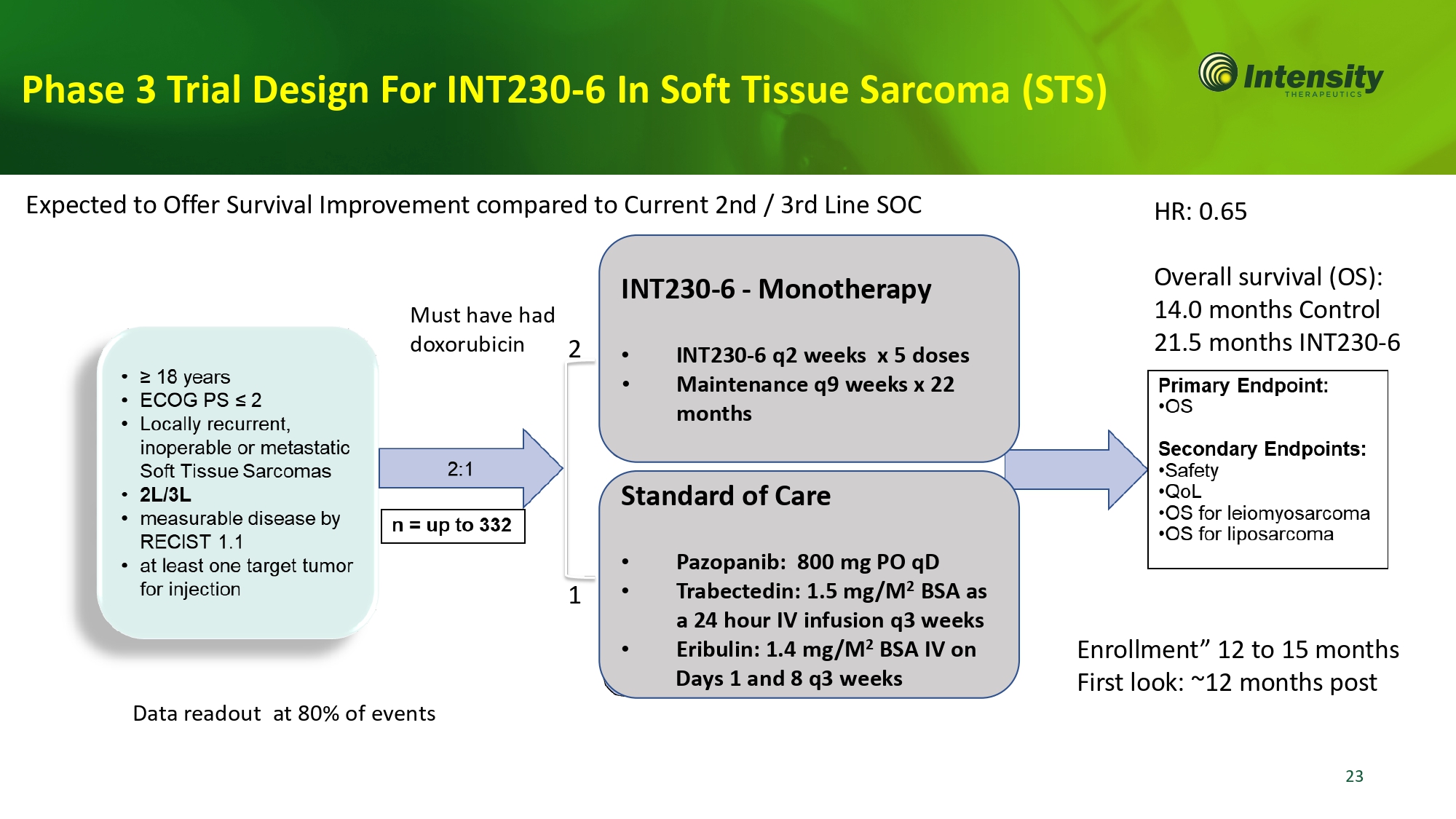
Must have had doxorubicin HR: 0.65 Overall survival (OS): 14.0 months Control 21.5 months INT230 - 6 Data readout at 80% of events Phase 3 Trial Design For INT230 - 6 In Soft Tissue Sarcoma (STS) INT230 - 6 - Monotherapy • INT230 - 6 q2 weeks x 5 doses • Maintenance q9 weeks x 22 months Standard of Care 23 • Pazopanib: 800 mg PO qD • Trabectedin: 1.5 mg/M 2 BSA as a 24 hour IV infusion q3 weeks • Eribulin: 1.4 mg/M 2 BSA IV on Days 1 and 8 q3 weeks 2 1 Enrollment” 12 to 15 months First look: ~12 months post Expected to Offer Survival Improvement compared to Current 2nd / 3rd Line SOC

• INT230 - 6 represents a new treatment approach to solid tumors (diffusion based immunological cell killing) for metastatic disease and presurgical (neoadjuvant) settings • Dose set by the total tumor burden – more personalized and spares the patient • Strong interest from: academic hospitals, major clinical oncology societies, big pharma and government • INT230 - 6 has: • induced significant necrosis in large tumors following a single dose • Immune activation observed of non - immunogenic cancer types • Shown favorable safety and promising increased survival efficacy 24 Summary

INTENSITY THERAPEUTICS 25 A NEW WEAPON IN THE WAR ON CANCER Investor Relations Contact: Argot Partners Jonathan Nugent Intensity@argotpartners.com Media Contact: Argot Partners David Rosen david.rosen@argotpartners.com Thank you!