00015672642023FYfalse0.5Subsequent EventsThe Company evaluated subsequent events from the balance sheet date through March 14, 2024, the date which the financial statements were issued.
00015672642023-01-012023-12-3100015672642023-06-30iso4217:USD00015672642024-03-01xbrli:shares0001567264intensity:StockIncentivePlan2021Member2023-12-3100015672642023-12-3100015672642022-12-31iso4217:USDxbrli:shares0001567264intensity:SeriesBConvertiblePreferredStockMember2022-12-310001567264intensity:SeriesBConvertiblePreferredStockMember2023-12-310001567264intensity:SeriesCConvertiblePreferredStockMember2022-12-310001567264intensity:SeriesCConvertiblePreferredStockMember2023-12-3100015672642022-01-012022-12-3100015672642021-12-310001567264us-gaap:PreferredStockMemberintensity:SeriesBConvertiblePreferredStockMember2021-12-310001567264us-gaap:PreferredStockMemberintensity:SeriesCConvertiblePreferredStockMember2021-12-310001567264us-gaap:CommonStockMember2021-12-310001567264us-gaap:AdditionalPaidInCapitalMember2021-12-310001567264us-gaap:RetainedEarningsMember2021-12-310001567264us-gaap:AdditionalPaidInCapitalMember2022-01-012022-12-310001567264us-gaap:RetainedEarningsMember2022-01-012022-12-310001567264us-gaap:PreferredStockMemberintensity:SeriesBConvertiblePreferredStockMember2022-12-310001567264us-gaap:PreferredStockMemberintensity:SeriesCConvertiblePreferredStockMember2022-12-310001567264us-gaap:CommonStockMember2022-12-310001567264us-gaap:AdditionalPaidInCapitalMember2022-12-310001567264us-gaap:RetainedEarningsMember2022-12-310001567264us-gaap:CommonStockMember2023-01-012023-12-310001567264us-gaap:AdditionalPaidInCapitalMember2023-01-012023-12-310001567264us-gaap:AdditionalPaidInCapitalMemberus-gaap:WarrantMember2023-01-012023-12-310001567264us-gaap:WarrantMemberintensity:ConversionOfPreferredStockIntoCommonStockMember2023-01-012023-12-310001567264us-gaap:PreferredStockMemberintensity:SeriesBConvertiblePreferredStockMember2023-01-012023-12-310001567264us-gaap:PreferredStockMemberintensity:SeriesCConvertiblePreferredStockMember2023-01-012023-12-310001567264us-gaap:CommonStockMemberintensity:ConversionOfPreferredStockIntoCommonStockMember2023-01-012023-12-310001567264us-gaap:AdditionalPaidInCapitalMemberintensity:ConversionOfPreferredStockIntoCommonStockMember2023-01-012023-12-310001567264intensity:ConversionOfPreferredStockIntoCommonStockMember2023-01-012023-12-310001567264us-gaap:CommonStockMemberintensity:ConversionOfConvertibleNotesToCommonStockMember2023-01-012023-12-310001567264us-gaap:AdditionalPaidInCapitalMemberintensity:ConversionOfConvertibleNotesToCommonStockMember2023-01-012023-12-310001567264intensity:ConversionOfConvertibleNotesToCommonStockMember2023-01-012023-12-310001567264us-gaap:RetainedEarningsMember2023-01-012023-12-310001567264us-gaap:PreferredStockMemberintensity:SeriesBConvertiblePreferredStockMember2023-12-310001567264us-gaap:PreferredStockMemberintensity:SeriesCConvertiblePreferredStockMember2023-12-310001567264us-gaap:CommonStockMember2023-12-310001567264us-gaap:AdditionalPaidInCapitalMember2023-12-310001567264us-gaap:RetainedEarningsMember2023-12-310001567264us-gaap:IPOMember2023-07-050001567264us-gaap:IPOMember2023-07-052023-07-050001567264us-gaap:OverAllotmentOptionMember2023-07-070001567264us-gaap:OverAllotmentOptionMember2023-07-072023-07-070001567264intensity:SeriesBConvertiblePreferredStockMember2023-04-300001567264intensity:SeriesARedeemablePreferredStockMember2023-04-3000015672642023-04-300001567264intensity:SeriesCConvertiblePreferredStockMember2023-04-3000015672642023-04-012023-04-30xbrli:pure0001567264intensity:SeriesARedeemablePreferredStockMember2023-01-012023-12-310001567264intensity:SeriesARedeemablePreferredStockMember2022-01-012022-12-310001567264intensity:SeriesBConvertiblePreferredStockMember2023-01-012023-12-310001567264intensity:SeriesBConvertiblePreferredStockMember2022-01-012022-12-310001567264intensity:SeriesCConvertiblePreferredStockMember2023-01-012023-12-310001567264intensity:SeriesCConvertiblePreferredStockMember2022-01-012022-12-310001567264us-gaap:EmployeeStockOptionMember2023-01-012023-12-310001567264us-gaap:EmployeeStockOptionMember2022-01-012022-12-310001567264us-gaap:WarrantMember2023-01-012023-12-310001567264us-gaap:WarrantMember2022-01-012022-12-310001567264us-gaap:ConvertibleDebtMemberintensity:A2021ConvertibleNoteMember2021-09-300001567264us-gaap:ConvertibleDebtMemberintensity:A2022ConvertibleNoteMember2022-11-300001567264us-gaap:ConvertibleDebtMemberintensity:A2023ConvertibleNoteMember2023-05-310001567264us-gaap:ConvertibleDebtMember2021-12-310001567264us-gaap:ConvertibleDebtMemberintensity:A2022ConvertibleNoteMember2022-01-012022-12-310001567264us-gaap:ConvertibleDebtMember2022-12-310001567264us-gaap:ConvertibleDebtMemberintensity:A2023ConvertibleNoteMember2023-01-012023-12-310001567264us-gaap:ConvertibleDebtMember2023-01-012023-12-310001567264us-gaap:ConvertibleDebtMember2023-12-310001567264us-gaap:ConvertibleDebtMember2023-07-052023-07-050001567264us-gaap:IPOMember2023-06-292023-06-290001567264us-gaap:IPOMember2023-06-290001567264us-gaap:IPOMember2023-07-070001567264us-gaap:CommonStockMember2023-06-292023-06-290001567264intensity:SeriesARedeemablePreferredStockMember2023-06-290001567264intensity:SeriesBConvertiblePreferredStockMember2023-06-290001567264intensity:SeriesCConvertiblePreferredStockMember2023-06-290001567264intensity:SecurityAgreementAdditionalStockMemberintensity:SeriesBConvertiblePreferredStockMember2023-06-292023-06-290001567264intensity:SecurityAgreementAdditionalStockMemberintensity:SeriesCConvertiblePreferredStockMember2023-06-292023-06-290001567264us-gaap:ConvertibleDebtMember2023-06-292023-06-290001567264intensity:StockIncentivePlan2021Member2021-12-310001567264intensity:StockOptionPlan2013Member2021-12-310001567264intensity:StockIncentivePlan2021Member2022-01-010001567264intensity:StockIncentivePlan2021Member2021-01-012021-12-310001567264us-gaap:ResearchAndDevelopmentExpenseMember2023-01-012023-12-310001567264us-gaap:ResearchAndDevelopmentExpenseMember2022-01-012022-12-310001567264us-gaap:GeneralAndAdministrativeExpenseMember2023-01-012023-12-310001567264us-gaap:GeneralAndAdministrativeExpenseMember2022-01-012022-12-310001567264us-gaap:EmployeeStockOptionMembersrt:MinimumMember2023-12-310001567264srt:MaximumMemberus-gaap:EmployeeStockOptionMember2023-12-310001567264us-gaap:EmployeeStockOptionMember2022-12-310001567264us-gaap:EmployeeStockOptionMember2023-01-012023-12-310001567264us-gaap:EmployeeStockOptionMember2022-01-012022-12-310001567264us-gaap:EmployeeStockOptionMembersrt:MinimumMember2023-01-012023-12-310001567264srt:MaximumMemberus-gaap:EmployeeStockOptionMember2023-01-012023-12-310001567264us-gaap:EmployeeStockOptionMembersrt:MinimumMember2022-01-012022-12-310001567264srt:MaximumMemberus-gaap:EmployeeStockOptionMember2022-01-012022-12-310001567264us-gaap:WarrantMembersrt:MinimumMember2023-12-310001567264srt:MaximumMemberus-gaap:WarrantMember2023-12-310001567264us-gaap:WarrantMember2022-12-310001567264us-gaap:WarrantMember2023-01-012023-12-310001567264us-gaap:WarrantMember2022-01-012022-12-310001567264us-gaap:WarrantMembersrt:MinimumMember2023-01-012023-12-310001567264srt:MaximumMemberus-gaap:WarrantMember2023-01-012023-12-310001567264us-gaap:WarrantMember2023-12-310001567264us-gaap:WarrantMemberintensity:UnderwriterMember2023-01-012023-12-3100015672642017-01-31utr:sqft00015672642017-02-2800015672642023-07-310001567264us-gaap:RelatedPartyMember2023-01-012023-12-310001567264us-gaap:RelatedPartyMember2022-01-012022-12-310001567264us-gaap:RelatedPartyMember2023-10-310001567264us-gaap:DomesticCountryMember2023-12-310001567264us-gaap:StateAndLocalJurisdictionMember2023-12-310001567264us-gaap:DomesticCountryMember2022-12-310001567264us-gaap:StateAndLocalJurisdictionMember2022-12-31 UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
(Mark one)
x ANNUAL REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2023
or
o TRANSITION REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ______________ to ______________
Commission File Number 001-41109
INTENSITY THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| | | | | | | | |
| Delaware | | 46-1488089 |
| (State or other jurisdiction of | | (I.R.S. Employer |
| incorporation or organization) | | Identification No.) |
| | | |
1 Enterprise Drive, Suite 430 | | |
Shelton, CT | | 06484-4779 |
| (Address of principal executive offices) | | (Zip Code) |
(203) 221-7381
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of Each Class | | Trading Symbol | | Name of Each Exchange on Which Registered |
| Common Stock, $0.0001 par value | | INTS | | The Nasdaq Stock Market |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | |
Large accelerated filer o | Accelerated filer o | Non-accelerated filer x | Smaller reporting company x | Emerging growth company x |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. o
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. o
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. o
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act of 1934). Yes o No x
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the Registrant, based on the closing price of the shares of common stock on The Nasdaq Stock Market on June 30, 2023, was $22.0 million.
As of March 1, 2024, the registrant had 13,709,377 shares of common stock, $0.0001 par value, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.
TABLE OF CONTENTS
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this report, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “will,” “project,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among other things, statements about:
•the initiation, timing, progress and results of future preclinical studies and clinical trials, and our research and development programs;
•our need to raise additional funding before we can expect to generate any revenues from product sales;
•our plans to develop and commercialize our product candidates;
•the timing or likelihood of regulatory filings and approvals;
•the ability of our research to generate and advance additional product candidates;
•the implementation of our business model, strategic plans for our business, product candidates and technology;
•our commercialization, marketing and manufacturing capabilities and strategy;
•the rate and degree of market acceptance and clinical utility of our system;
•our competitive position;
•our intellectual property position;
•developments and projections relating to our competitors and our industry;
•our ability to maintain and establish collaborations or obtain additional funding;
•our expectations related to the use of our cash and cash equivalents and investments; and
•our estimates regarding expenses, future revenue, capital requirements and needs for additional financing.
These forward-looking statements reflect our management’s beliefs and views with respect to future events and are based on estimates and assumptions as of the date of this Annual Report on Form 10-K and are subject to risks and uncertainties. We discuss many of these risks in greater detail under “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. Given these uncertainties, you should not place undue reliance on these forward-looking statements.
You should read this Annual Report on Form 10-K and the documents that we have filed with the SEC as exhibits to this Annual Report on Form10-K and with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect. We qualify all forward-looking statements in this Annual Report on Form 10-K by these cautionary statements. Except as required by law, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events, or otherwise.
PART I
Item 1. Business
OVERVIEW
Intensity Therapeutics, Inc. is a late-stage clinical biotechnology company passionately committed to applying scientific leadership in the field of localized cancer reduction leading to anti-cancer immune activation. Our new approach involves the direct injection into tumors of a unique product created from our DfuseRxSM discovery platform.
Intratumoral (“IT”) treatment, or treatment designed to contain a drug inside a tumor without spreading to the rest of the body, has been an objective of clinicians since discovery of chemotherapeutic agents. The challenge with IT treatment approaches is that a tumor’s lipophilic, high fat, dense and pressurized microenvironment is incompatible with and does not absorb water-based products. We believe that this drug delivery challenge limits the effectiveness of prior and current IT treatments, which involve injecting aqueous drugs into a tumor without sufficient consideration of the tumor environment (regardless of the drug’s mechanism or approach, i.e. the stimulation of an inflammatory response or efforts to attract immune cells into a hostile live tumor). Accordingly, there remains a continued unmet need for the development of direct IT therapies for solid tumors that provide high local killing efficacy coupled with nontoxic systemic anti-cancer effects. We believe we have created a product candidate with the necessary chemistry to overcome this local delivery challenge. Evidence shows the mechanism of tumor killing achieved by our drug candidate also leads to systemic immune activation and T-cell repertoire expansion in certain cancers.
Our platform creates patented anti-cancer product candidates comprising active anti-cancer agents and amphiphilic molecules. Amphiphilic molecules have two distinct components: one part is soluble in water and the other is soluble in fat or oils. When an amphiphilic compound is mixed with therapeutic agents, such as chemotherapies, the agents also become soluble in both fat and water. Our product candidates include novel formulations consisting of potent anti-cancer drugs mixed together with these amphiphilic agents.
Our lead product candidate, INT230-6, is primarily comprised of three components: (i) cisplatin, a proven anti-cancer cytotoxic agent, (ii) vinblastine sulfate, also a proven anti-cancer cytotoxic agent, and (iii) an amphiphilic molecule (“SHAO”) which enables the two cytotoxic agents to disperse through a tumor and diffuse into cancer cells following a direct intratumoral injection. These three components are mixed and combined into one vial at a fixed ratio. Cisplatin and vinblastine sulfate are both generic and available to purchase in bulk supply commercially. The United States Food & Drug Administration (the “FDA”) has approved both drugs as intravenous agents for several types of cancers. Cisplatin was first approved in 1978 for testicular cancer, and is also approved in ovarian and bladder cancer. The drug is also used widely in several other cancers including pancreatic and bile duct cancer. Vinblastine sulfate was first approved in 1965, and is also approved in generalized Hodgkin’s disease, lymphocytic lymphoma, advanced carcinoma of the testis, and certain types of sarcoma. The drug is also used in breast and lung cancer.
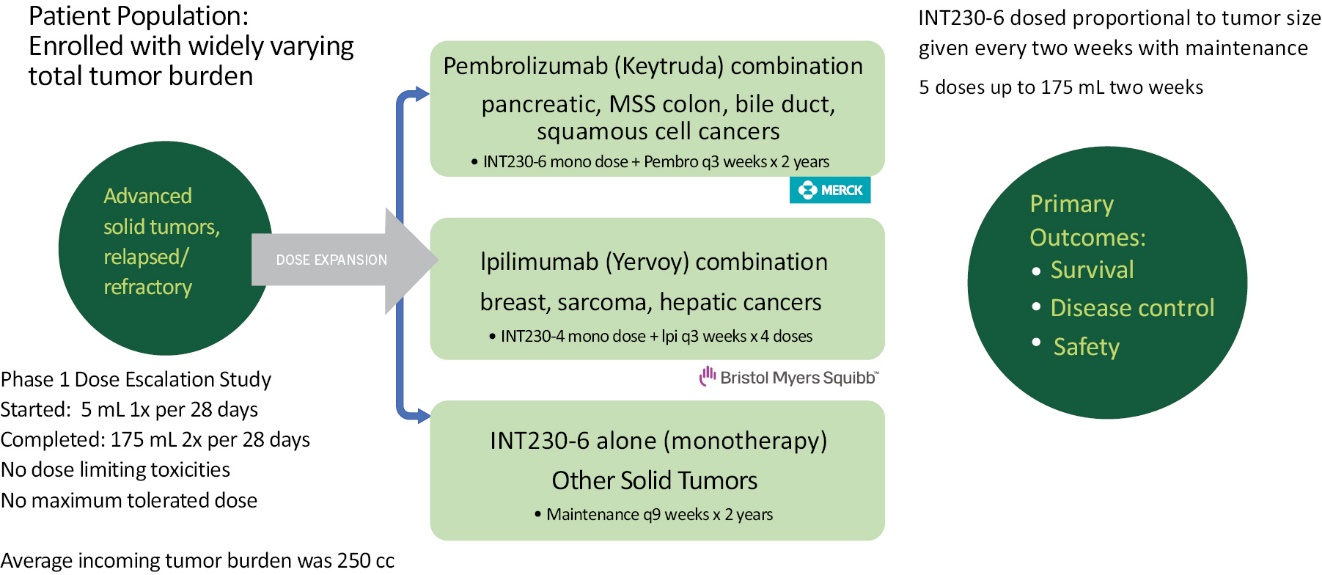
In 2017, we initiated a Phase 1/2 dose escalation study (“IT-01”) using INT230-6 in the United States under an investigational new drug application (“IND”) authorized by the FDA and in Canada under a preclinical trial application (“CTA”) approved by Health Canada (“HC”). The study tested the safety and efficacy of INT230-6 in patients with refractory or metastatic cancers, and enrolled 110 patients in three arms: (i) INT230-6 used as a monotherapy, (ii) INT230-6 in combination with Merck’s Keytruda® (pembrolizumab), and (iii) INT230-6 in combination with Bristol Myers Squibb’s (“BMS”) Yervoy® (ipilimumab). We completed enrollment of IT-01 in June 2022, locked the IT-01 database in February 2023 and finalized the clinical study report in September 2023. We delivered the combination-specific reports and other information to our partners in the fourth quarter of 2023.
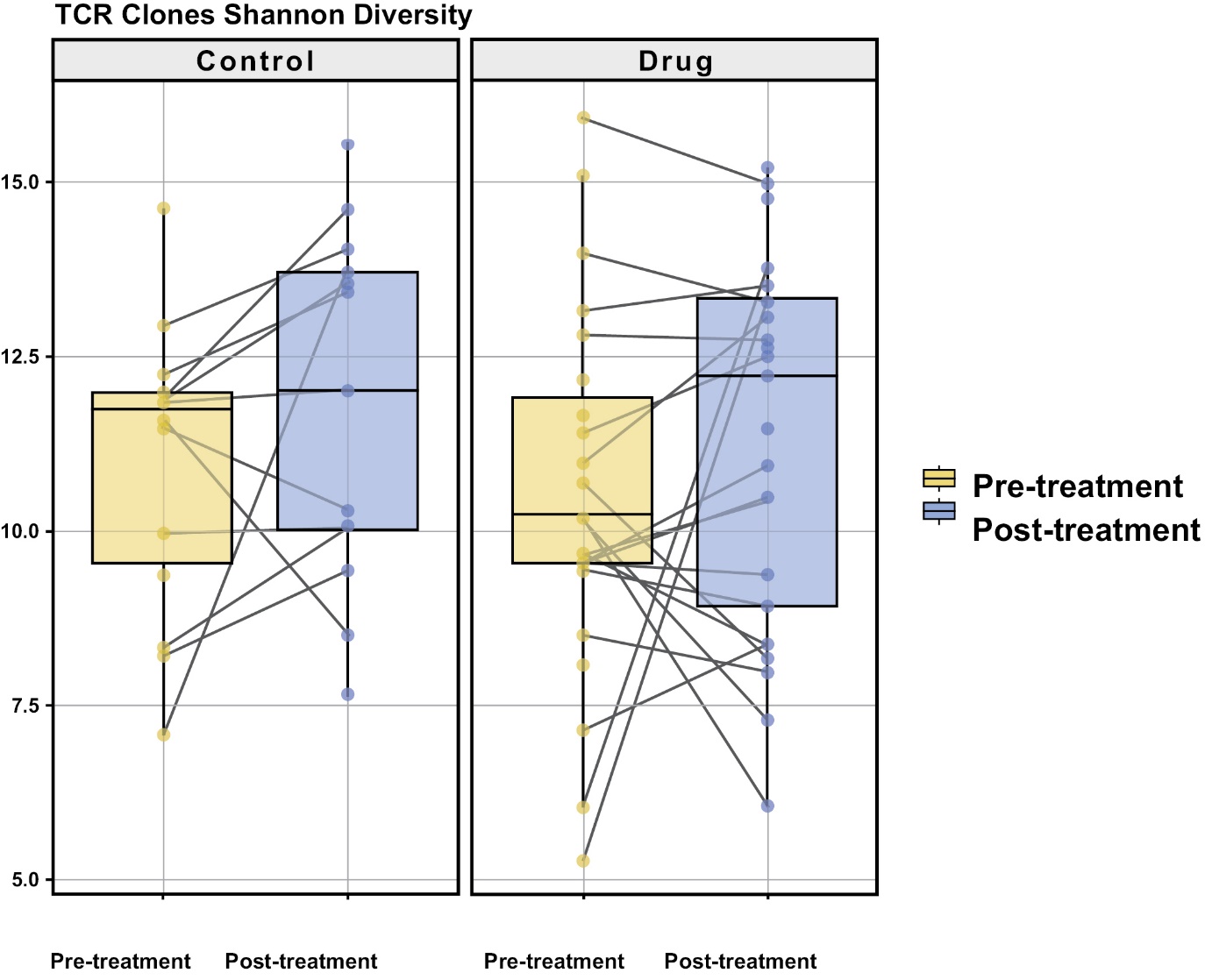
In 2021, we initiated a Phase 2 randomized study that tested INT230-6 as a monotherapy treatment in early-stage breast cancer for patients not suitable for presurgical chemotherapy (the “INVINCIBLE-2 Study” or “IT-02”). The study enrolled 91 subjects and the database was locked in November 2023. The key endpoint was whether INT230-6 could reduce a patient’s cancer compared to no treatment, which is the current standard of care (“SOC”), or a saline injection. Substantial reduction of cancer presurgically in aggressive forms of cancer has been shown to correlate with delaying disease recurrence. Other endpoints of the INVINCIBLE 2 Study were to understand the percentage of necrosis that can be achieved in tumors for a given dose, especially tumors larger than 2 centimeters in longest diameter, and whether either a local or whole-body anti-cancer immune response could be induced. The INVINCIBLE-2 Study demonstrated a high order of necrosis in presurgical breast cancer tumors in the period from diagnosis to surgery, with some patients experiencing greater than 95% necrosis of the tumor. Data from the INVINCIBLE-2 Study demonstrated that INT230-6 had a favorable safety profile. An increase of certain types of immune cells (CD4+ and NK T-cells) in the tumor and blood was also shown. Additionally, there was an increase in the T-cells repertoire relative to control.
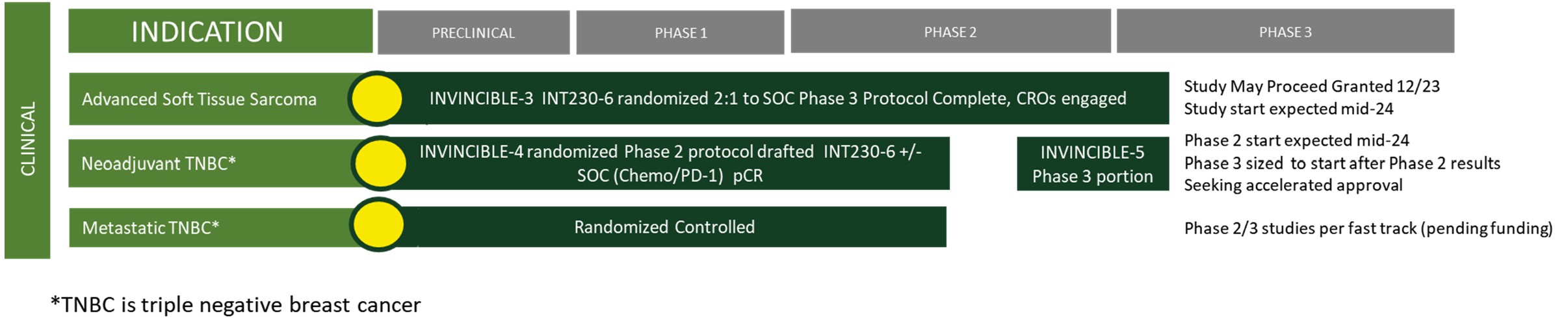
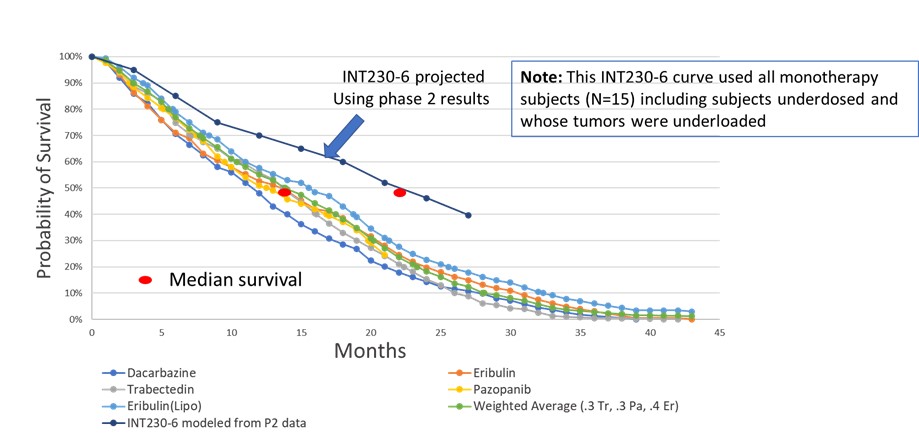
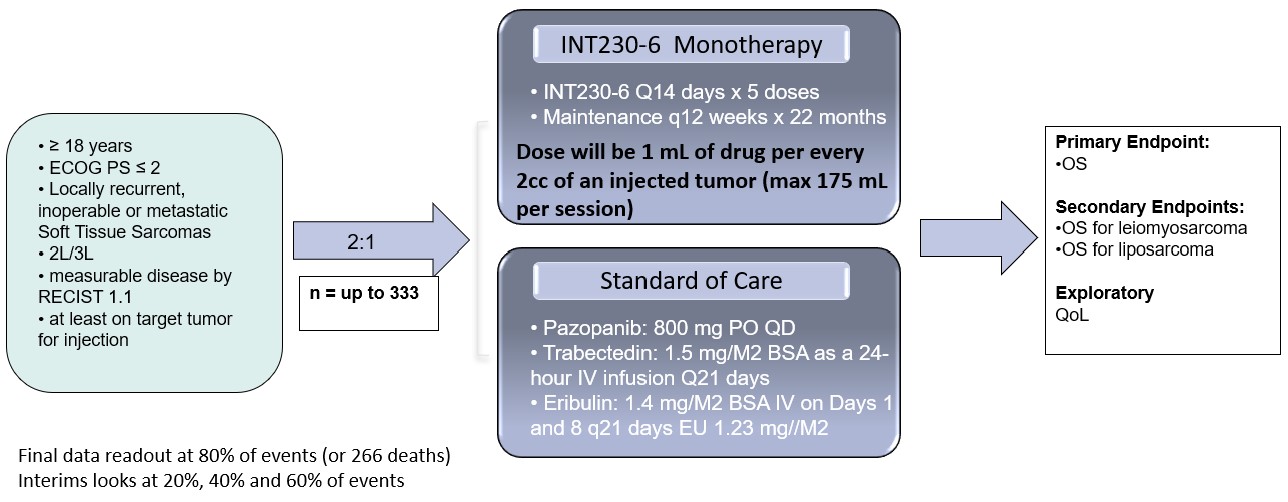
In mid-2024, we intend on initiating a Phase 3 open-label, randomized study testing the superiority INT230-6 used as monotherapy compared to the standard of care drugs in 2nd and 3rd line treatment for certain soft tissue sarcoma subtypes (the “INVINCIBLE-3 Study” or “IT-03”). We plan to enroll 333 patients with an endpoint of overall survival.
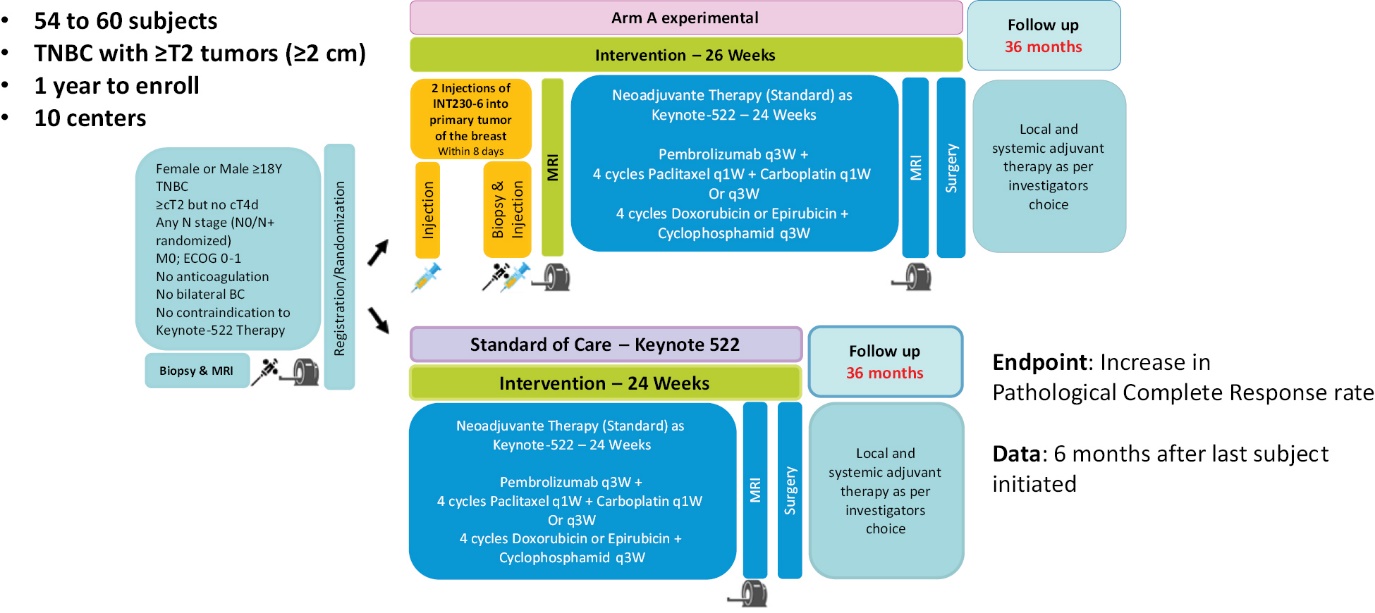
Also in mid-2024, we intend on initiating a Phase 2/3 program testing INT230-6 in combination with the SOC treatment (chemotherapy/immunotherapy) compared to SOC alone in women with triple negative breast cancer in presurgical (neoadjuvant) breast cancer (“IT-04”). The endpoint for the Phase 2 portion of the trial is the change in the pathological complete response rate for the combination compared to the SOC alone. We expect to begin the Phase 2 portion of the study in mid-2024, which will provide data to size the Phase 3 portion of the study.
We have also successfully developed Phase 3 quality analytical methods for the three INT230-6 components and successfully manufactured a large-scale batch of INT230-6. In a meeting with the FDA in the fourth quarter of 2023, we agreed on a chemical manufacture and control (“CMC”) plan for Phase 3 and product registration for our three key ingredients and INT230-6. If we successfully execute the agreed upon plan, the CMC portion of a New Drug Application (“NDA”) should be acceptable to the FDA for product approval and registration (subject to final NDA review).
Our Pipeline
Our pipeline is focused on realizing the full potential of INT230-6 in metastatic and local disease settings to help cancer patients with major unmet medical need. We are exploring the use of INT230-6 across multiple cancer types (including those types that do not normally respond to immunotherapy) and “hot” tumors (cancer types that are more likely to respond to immunotherapy).
Based on data generated in IT-01 and the INVINCIBLE-2 studies, our current forward pipeline consists of:
•A Phase 3 open-label, randomized study testing the superiority INT230-6 used as monotherapy compared to the standard of care drugs in 2nd and 3rd line treatment for certain soft tissue sarcoma subtypes. For every three patients enrolled, two will receive INT230-6 and one will receive SOC drug(s) chosen by the investigators depending on the type of sarcoma. The Company is working with several contracted vendors to initiate the Phase 3 trial. We plan to enroll 333 patients with an endpoint of overall survival. In September 2023, we announced that the FDA granted orphan drug designation for the treatment of soft tissue sarcoma to the three active moieties comprising INT230-6: cisplatin, vinblastine sulfate, and the diffusion enhancer SHAO.
•A Phase 2/3 study testing INT230-6 in combination with the SOC treatment (chemotherapy/immunotherapy) compared to the SOC alone in women with triple negative breast cancer in presurgical (neoadjuvant) breast cancer. The endpoint for the Phase 2 portion of the trial is the change in the pathological complete response rate for the combination compared to the SOC alone.
•A Phase 2/3 clinical study in metastatic triple negative breast cancer, contingent on raising additional capital to fund the study. In 2018, we received Fast Track Designation by the FDA to use INT230-6 in metastatic triple negative breast cancer for patients whose cancer has progressed following one or two prior drug treatments.
In addition, we plan to continue to research and test new product candidates with improved immune activating properties. Through research studies conducted in animals, we have identified a promising product candidate currently designated as INT33X. We believe that the INT33X product candidate development program will most likely lead to the creation of new patents and other intellectual property. As part of our development program for INT33X, we will first conclude our on-going research studies in mice, after which we will finalize the exact product candidate composition before proceeding with clinical development.
Our Partnerships
•The National Cancer Institute (NCI). In May 2014, we were awarded a Collaboration Research and Development Agreement (CRADA) by the National Institute of Health’s National Cancer Institute. The research sought to understand the mechanism of action of INT230-6 and test the drug in several models in the NCI’s laboratories. The program resulted in a peer-reviewed publication titled Intratumorally delivered formulation, INT230-6, containing potent anti-cancer agents induces protective T-cell immunity and memory, which appeared in the journal OncoImmunology 2019 Vol 8 No 10; 15 and that was jointly authored by us and the NCI. The data for the paper was generated entirely by the NCI in their laboratories and reported the critical role of T-cells in promoting complete tumor regression using our drug candidate and that INT230-6 was synergistic with anti-PD-1 (programmed death receptor 1) and anti-Cytotoxic T Lymphocyte-Associated Antigen 4 (CTLA-4) antibodies.
•Merck. In June 2019, we entered into an agreement with Merck to evaluate the combination of INT230-6 with Keytruda® (pembrolizumab), Merck’s anti-PD-1 therapy, in patients with advanced solid malignancies, including pancreatic, bile duct, squamous cell and non-MSI high colon cancers. In our IT-01 study, we treated 30 patients with this combination arm. After nearly two years of dosing a combination of Keytruda and INT230-6, patients showed comparable safety to INT230-6 monotherapy. In addition, only three grade 3 immune-related adverse events reported in patients receiving the combination of INT230-6 with Keytruda. We completed study dosing in December 2022, finalized the clinical study report, tables, listings and figures for the Keytruda cohorts and provided the study information to Merck in December 2023.
•Bristol Myers Squibb. In April 2020, we entered into an agreement with BMS to evaluate the safety and efficacy of INT230-6 with Yervoy® (ipilimumab), BMS’s CTLA-4 immune checkpoint inhibitor, in patients with breast, liver, and advanced sarcoma cancer. In our IT-01 study, we treated 18 patients in this combination arm, and there was only one grade 3 immune-related adverse event (colitis) reported. We completed study dosing in December 2022, finalized the clinical study report, tables, listings and figures for the Yervoy cohorts and provided the study information to BMS in December 2023.
Our Clinical Data
INT230-6 has already generated anti-cancer evidence of activity as a single agent in clinical studies. Localized and abscopal effects have been observed in several patients. Tumor regressions with killing of the cancer cells is widely observed in injected lesions. Many patients who have exhausted all approved treatments for their types of cancer benefited from our product candidate. Our clinicians have reported tumor stabilization, tumor shrinkage, long periods without new tumors forming, size reductions of uninjected tumors and a reduction in disease symptoms. These results have been observed in combination with lower toxicities over a period of several months and post-treatment.
•Increased Survival Observed in Metastatic Disease. Preliminary data presented at the American Society of Clinical Oncology (ASCO) the Society for Immunotherapy of Cancer (SITC) and the Connective Tissue Oncology Society (“CTOS”) for sarcoma in 2022 and 2023 indicated that patients receiving INT230-6 appear to live longer compared to historical data for subjects in phase 1/2 sarcoma studies.
•Acceptable Safety Profile of the New Drug/Treatment Approach to Date. During the IT-01 study there were 820 injections of INT230-6 into 238 tumors, including 502 injections into visceral tumors deep in the body. Injection locations include the pancreas, liver, lung, and lymph nodes. No maximum tolerated dose had been reached. In our study IT-01 in metastatic patients, most adverse events were minor grade 1 or 2; a total of 15 patients out of 110 (13.6%) had a grade 3 even related to the drug regimen (INT230-6 alone or combined with the two immunotherapies). The primary grade 3 events were pain, anemia, rash, fatigue vomiting, dehydration and dizziness. There was 1 laboratory-based grade 4 adverse event that resolved quickly, a decrease in the number of neutrophils, the most common type of white blood cell that contributes toward the healing of damaged tissues and resolving infections. There were no grade 5 adverse events. Please see Table 1 and 2 in the “Results from IT-01 Phase 1/2 Clinical Trial” portion of our “Business” section on page 67 of this report for more information. We believe the safety profile consisted of mainly low grade related adverse events because the drug primarily stays in the tumor and the potent agents did not travel throughout the body. Measurement of the
amount of the drugs seen in the blood (pharmacokinetics or PK) indicated that more than 95% of the drug that was dosed remained in the tumor.
Our Manufacturing Capabilities
We work with clinical manufacturing organizations. In the fourth quarter of 2023, we successfully developed the Phase 3 quality analytical methods for measurement of the key INT230-6 components, validated those methods and manufactured our fourth current Good Manufacturing Practice (“cGMP”) clinical batch of the drug product that met specifications. During the fourth quarter, the Company requested and was granted a meeting that was held with the FDA to review the INT230-6 CMC for INT230-6. The CMC discussion focused on the tasks necessary to initiate the Phase 3 study and future product registration as part of a potential New Drug Application (NDA). During the meeting, the Company and the FDA agreed upon a plan for the CMC set of activities for the active pharmaceutical ingredients and the drug product (INT230-6) necessary for the NDA.
To commence the Phase 3 sarcoma program, we engaged contract research organizations to help manage the Phase 3 sarcoma study. We believe that INVINCIBLE-3 will be the first local therapy (though with systemic immune activating properties) to be tested as a single agent in metastatic disease compared to active SOC IV or oral agents.
Our Proprietary Drug Discovery platform, DfuseRxSM
Since our inception, we have conducted research using our discovery platform. Our technology platform allows us to identify novel product formulations and test the products’ activity in animal or test tube models of cancer. Using our platform technology, we evaluated several potential formulations comprising various amphiphilic molecules that act as cancer cell penetration enhancers. We tested formulations using our technology with many potent, anti-cancer drugs (with different mechanisms of action) in various combinations under several conditions to discover our lead product candidate, INT230-6.
Our Strategy
We believe our treatment approach may overcome some of the inherent problems of treating cancer with less toxicity. We intend to apply our deep understanding of our novel drug delivery technology to create a range of new direct killing and immune-activating products candidates while focusing on our lead clinical programs. If successful, we hope to fundamentally change the way cancer is treated for multiple cancer types in both the metastatic and presurgical disease settings.
We seek to build a company that develops and commercializes a new medicine and treatment methodology. By applying a disciplined focus on product development, we seek to transform the lives of cancer patients and change the very essence of cancer treatment.
Our objective is for patients to overcome their cancer without harm, to live a long life with high quality and to eliminate the fear of disease recurrence. We maintain a culture of high integrity that embraces the patient and their caregivers. A simple strategy: taking care of the patient will benefit all stakeholders.
Key elements of our strategy include:
•To focus our resources to aggressively pursue the research and development of our novel medicine to transform patient lives.
•To always remember that taking care of and benefiting the patient is the most important element to being successful.
•To effectively manage costs by outsourcing research and development to qualified, academic, private or government laboratories to leverage third-party expertise, while maintaining internal know-how, expertise and intellectual property.
•To build an internal team of experienced industry veterans that can work independently and who know how to get the product development job done.
•To create a large body of rigorous data, publications, presentations, collaborations and training materials about the new product candidates.
•To continuously communicate to the medical community and patients of the power of our new approach.
•To continue our commitment to precision medicine and personalized care for each and every patient.
•To assure that our technology is fully understood, explored, and used as designed.
Market Opportunities for Our Product Candidates
The development of a tumor is a complex biological process involving uncontrolled cellular division and growth. Cancer arises from mutations in our own cells. When such cellular alterations happen the immune system often cannot distinguish between cancer and healthy cells. Cancer cells adapt to evade and thwart immune cells in several ways and can thus grow unchecked.
According to the American Cancer Society, in 2024 there will be an estimated 2 million new cancer cases diagnosed and over 611,000 cancer deaths in the United States. Cancer is the second most common cause of death in the U.S. after heart disease. According to the American Society of Clinical Oncology’s journal, the ASCO Post, the national cost of cancer care in the United States is expected to rise to $246 billion by 2030. As healthcare costs in general continue to escalate, expenses due to cancer are a major contributing factor.
Metastatic Disease
The overwhelming, unmet medical need is better treatment of solid tumors; 90% of cancer patient deaths are due to solid tumors. Unfortunately, even with the best new therapeutic agents, the long-term survival rates for inoperable or metastatic cancer are extremely low (often single digits) and toxicity (the collateral damage to the patient’s health) is debilitating.
Five-year Survival Percentage Rates for Metastatic, Late-Stage Cancers
| | | | | | | | | | | | | | | | | | | | |
Cancer type | | 5 Year
Survival
(%)* | | Cancer type | | 5 Year
Survival
(%)* |
Breast | | 29 | | Ovarian | | 30 |
Colon/rectal | | 15 | | Pancreas | | 3 |
Esophagus | | 5 | | Prostate | | 30 |
Kidney | | 14 | | Sarcoma | | 16 |
Larynx | | 34 | | Testis | | 95 |
Liver | | 3 | | Thyroid | | 53 |
Lung/Bronchus | | 6 | | Urinary bladder | | 6 |
Melanoma (skin) | | 30 | | Uterine cervix | | 18 |
Oral cavity | | 40 | | Uterine corpus | | 16 |
____________
*For cancers that have moved to distal sites
Data sources for the above table: Surveillance, Epidemiology, and End Results National Cancer Institute, SEER 5-Year Relative Survival Rates, 2011 – 2017
In late-stage, metastatic disease, tumors often become resistant to all therapies, even after the agents have provided some efficacy benefit. The reality today for many cancer types is that if the disease is detected late, most treatments are highly toxic and few of today’s approaches provide patients with much hope of long-term survival. Even with good outcomes, whether by surgical, chemical, radiative, immunological or ablative methods, cancer treatments are invasive, have severe side effects, damage the body and are mentally demanding on patients and their families.
Local Disease
Today, the annual number of interventional oncology procedures in the U.S. alone are estimated in the millions. For example, the majority of breast cancer tumors identified are local to the breast or are regional. As a result, there are 170,000 lumpectomies performed in the U.S. each year. Dr. Roshni Rao, Chief, Breast surgery program, at New York-Presbyterian/Columbia University Medical Center wrote in the Cancer Letter that “although lumpectomy is the best option for many breast cancer patients, with 170,000 procedures performed annually, it is not perfect. All too often, a post-operative pathology report shows that while the surgeon may have removed the entire tumor, a second surgical procedure is needed to clean up lingering cancer cells. Known as re-excision, it occurs in roughly 20% to 25% of cases, on average. It is
critical for surgeons and their patients to have access to the latest innovations, once demonstrated effective by clinical research, be used wherever and whenever possible.” Our drug candidate’s potential to kill cancer quickly prior to surgery and engage an anti-cancer immune response may provide a higher percentage of patients a greater five-year event-free survival for a number of tumor types.
Breast Cancer
About 1 in 8 U.S. women (about 13%) will develop invasive breast cancer over the course of her lifetime. In 2024, there will be an estimated 313,510 new cases of invasive breast cancer diagnosed in women; 2790 new cases diagnosed in men, and an additional 56,500 new cases of ductal carcinoma in situ diagnoses in women. (Siegel et al., 2024). Breast cancer is the most commonly diagnosed cancer among American women. Breast cancer became the most common cancer globally as of 2021, accounting for 12% of all new annual cancer cases worldwide, according to the World Health Organization.
Approximately 11 – 17% of breast cancers test negative for estrogen receptors, progesterone receptors, and excess human epidermal growth factor receptor 2 (HER2) protein, qualifying them as triple negative (“TNBC”). TNBC is considered to be more aggressive and have a poorer prognosis than other types of breast cancer, mainly because there are fewer available targeted medicines especially for women have tumors above 2 cm in longest diameter. Patients typically receive chemotherapy. According to a study published in the Journal of Clinical Oncology, patients who fail two lines of therapy for TNBC typically progress within nine weeks. Those who have failed three lines progress within four weeks.
Sarcoma
Soft tissue sarcoma is a broad term for cancers that start in soft tissues (muscle, tendons, fat, lymph and blood vessels, and nerves). These cancers can develop anywhere in the body but are found mostly in the arms, legs, chest, and abdomen. There are many types of soft tissue tumors, and not all of them are cancerous.
There are many types of sarcoma; however, the three most common are bone sarcoma (referred to as osteosarcoma), leiomyosarcoma, undifferentiated pleomorphic sarcoma and liposarcoma. Leiomyosarcoma is a type of sarcoma that grows in the smooth muscles. The smooth muscles are also in the hollow organs of the body, including the intestines, stomach, bladder, and blood vessels. In females, there is also smooth muscle in the uterus. When sarcoma is metastatic prognosis is poor; even with chemotherapy, half of people diagnosed with metastatic disease die within 15 months. Each year, 12,000 people in the U.S. and 1,150 in Canada are newly diagnosed with soft tissue sarcomas. About 3,000 patients have bone sarcomas.
Chemotherapy Treatment
There is a high unmet medical need for improved cancer treatments. Currently, early detection coupled with surgery and systemic chemotherapy is the most effective treatment against most cancers. For metastatic disease, systemic chemotherapy represents the backbone of care for many cancers. However, chemotherapeutic resistance often results in therapeutic failure and eventually death. Not only is chemotherapy often ineffective for cancers that exhibit such resistance, but this approach is also highly toxic for many patients (Cancer Cell Int. 2015; 15:71). Almost all current anti-cancer drug therapies load drug throughout the entire body including classic chemotherapy before surgery (neoadjuvant), after surgery (adjuvant), targeted therapy, antibodies or antibody drug conjugates, liposomal or nanoparticle delivered drugs. Many cancer cells in tumors are located away from blood vessels (referred to have hypoxic regions) and systemic administration of chemotherapy is ineffective at delivering the needed amounts of the medicine to all parts of the tumor. A significant limitation of the current chemical-based anti-cancer treatments is proper drug delivery. Another challenge for systemic approaches is poor absorption or cellular mechanisms in the cancer cell to remove the drugs.
Immunotherapy
There has been much excitement over the past decade about the promise of immunotherapy in treating cancer. These novel product candidates are designed to mobilize an immune system to attack cancer. The field of cancer immunotherapy has become the primary focus of treatment for many tumor types. There is significant interest from pharmaceutical companies, physicians and patients in advancing new, immune-based treatment concepts. Today, some patients with formerly fatal cancers are experiencing long term survival benefits with immune-based treatments. Immunotherapy has shown promise against the most mutated cancers such as melanoma, renal cell carcinoma, squamous cell carcinomas and subsets of lung cancers. Often these new immune stimulating drugs work in patients having high levels of specific markers,
such as the percentage of a protein on the surface of the cancer cell known as PD-L1 or the number of genetic mutations that may have caused the cancer referred to as a tumor’s mutational burden.
Many cancers, however, are also unresponsive to immunotherapy. Even for those cancers that are considered “immunogenic”, many patients are unresponsive. As a result, immunotherapy has not worked well for the majority of solid tumor types, including sarcoma, pancreatic cancer, colon cancer, triple negative breast cancer and brain cancer. At times, when using immunotherapies, the immune system has trouble distinguishing cancer from normal tissue and attacks healthy cells. Thus, the immune therapies induce side effects. To enable more patients to benefit from immunotherapy, new technologies that are able to improve recognition of the cancer by the immune system, or disrupt the tumor’s ability to evade immune cells, are critical and strongly needed.
Challenges Facing Current Treatments
We believe that an effective cancer treatment must overcome three major problems.
1.The diverse nature of the disease: In most patients, there are two populations of the cancer with different physical properties. The local component is comprised of the well-defined, visual large tumors, seen in x-ray or imaging scans, that invade organs and tissue. The systemic aspect is comprised of cells circulating or implanted throughout the body. Essentially, cancer is often simultaneously both microscopic (unseen) and macroscopic (radiographically seen).
2.Unreachable parts of tumors: Current systemic methods of delivering cancer drugs either orally or intravenously (IV) do not reach many portions of tumors due to a lack of blood supply. These areas are referred to as hypoxic (low oxygen) regions. These areas of the tumor can also impede the influx of immune cells. Intravenous or system dosing of cytotoxic agents suppresses the systemic immune system (Mathios et al, STM 2016) and reduces the potential of immunotherapies.
3.Lack of immune cell recognition and activation by tumor processes to evade: Immune cells have difficulty recognizing/distinguishing cancer cells from normal cells. Cancer also can cloak itself from the immune cells and create barriers to reduce their influx into the tumor.
Our Treatment Approach
Our treatment concept pioneers a new approach to treating cancer — kill tumors in the body (in situ) to create from the patient’s own cancer a recognizable, high-quality material (referred to as antigen) for better immune cell engagement against the cancer (immunological cell kill).
Our new concept uses a delivery molecule to enable the dispersal of potent drugs throughout the tumor that can also diffuse into the cancer cells. This process effectively loads the tumor with strong killing agents, which are retained within the cells. The active agents themselves used in our product candidate also have properties that improve immune recognition of the cancer. At the right dose our product candidates can completely saturate an injected tumor delivering high concentrations of drug into the cancer cells and killing the entire tumor. This process removes the cancer’s cloaking system, decreases the barriers to immune influx and activates a body-wide anti-cancer immune response to attack the uninjected tumors and unseen metastases. Our clinical data suggests that not all tumors need be injected for long term disease control.
Through our novel, drug treatment technology, we hope to transform the lives of patients with cancer. Our objectives are to increase patient longevity, reduce side effects, remove the fear of treatment, empower the patient, and minimize the risk of disease recurrence.
Our Lead Product Candidate: INT230-6
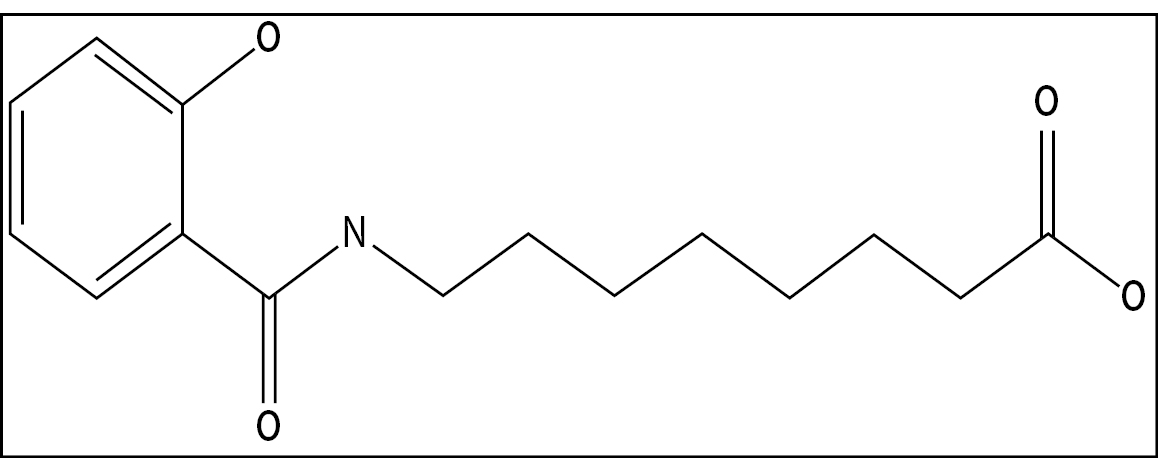
Our lead product candidate, INT230-6, is primarily comprised of three components: (i) cisplatin, a proven anti-cancer cytotoxic agent, (ii) vinblastine sulfate, also a proven anti-cancer cytotoxic agent, and (iii) SHAO, a penetration enhancing amphiphilic molecule. The SHAO chemical structure is shown in Figure 1 below. When injected into tumors, INT230-6 can kill the tumors. Our safety studies show that if the drug is (accidentally) injected into healthy tissue there is no observation of damage. The drug agents enter the blood stream at low doses. The unique amphiphilic SHAO compound formulated product candidate increases the dispersion of the drug throughout the tumor following intratumoral injection. Our technology is novel and unique. For those familiar with drug delivery technologies in cancer, it is important to understand that our product candidate is not a liposome, not a nanoparticle nor an emulsion. INT230-6 is a 100% water-
based formulation with tissue dispersion properties that do not destroy cancer cell membranes. We are unaware of any previous anti-cancer drug or prior intratumoral preparation with similar characteristics.
Figure 1 – Chemical Structure of SHAO
The SHAO molecule facilitates drug dispersion throughout the tumor. The molecule allows the tumor to absorb the killing agents and facilitates their diffusion into the cancer cells. Once in the cancer cell one drug cisplatin binds the DNA and causes the cell apoptosis (death) whereas the other agent, vinblastine sulfate, destroys the cell’s tubulin to shut down replication. Data in humans suggests that when administered at the proper drug dose to tumor volume ratio, a significant portion of the injected tumor can be killed on a single dose. In addition, there is evidence (in both animals and humans) that for certain cancers there is an activation of the immune system.
Our novel intratumoral (IT) technology is different than other IT approaches in four important ways:
1)We recognized that the composition of a tumor is highly unfavorable to direct injection of water-based products because the tumor has a high fat content and is under surrounding pressure. To be effective, an IT drug must disperse, be absorbed by the tumor and enter the cancer cell. Without our unique formulation chemistry water soluble drugs are not readily dispersed or absorbed by a tumor.
2)Our delivery technology is based on a proven science that uses amphiphilic molecules to transport drugs through tissue. The active drug agents in our lead product candidate (cisplatin and vinblastine sulfate) are established, commercial, potent killing agents with immune stimulating properties that as of now are only used as IV products. Both cisplatin and vinblastine sulfate have dual direct killing and immune activating mechanisms of action. Cisplatin binds to DNA to cause apoptotic cell death and also attracts and binds T-cells via TL9 receptors. Vinblastine sulfate destroys tubulin to stop replication and also induces dendritic cell maturation.
3)Unlike other IT products, our product candidates have multiple opportunities well beyond skin tumors, such as melanoma. Our lead product candidate, INT230-6, has shown the ability to kill tumors deep in the body such as in the liver, lung, and peritoneum. The product candidate has also demonstrated ability to kill tumors from several cancer types with abscopal effects and increased overall survival compared to historical results in Phase 1/2 studies.
4)Our product candidate has potential to kill tumors in a manner and could be used before surgery immediately after diagnosis or for treatment of cancers where there are no therapeutic agents or suitable local treatments available.
INT230-6 in Animals
Our first research studies in mice were conducted with organizations that provide services under contract, referred to as contract research organizations (“CROs”). Our Company collaborated with the Vaccine Branch of the National Cancer Institute (NCI) in Bethesda, MD. The research with the NCI was established after the National Institutes of Health awarded our Company a cooperative research and development agreement (CRADA). The program was quite successful and culminated with the publication of a paper in July 2019 that we jointly authored with the NCI. In that publication we reported that INT230-6 treatment resulted in regression from baseline in 100% of the tumors and complete response in up to 90%. Experiments showed a critical role of T-cells in promoting complete tumor regression. Mice with complete response were protected from subcutaneous and intravenous re-challenge of cancer cells. Thus, immunological T-cell memory was induced by INT230-6.
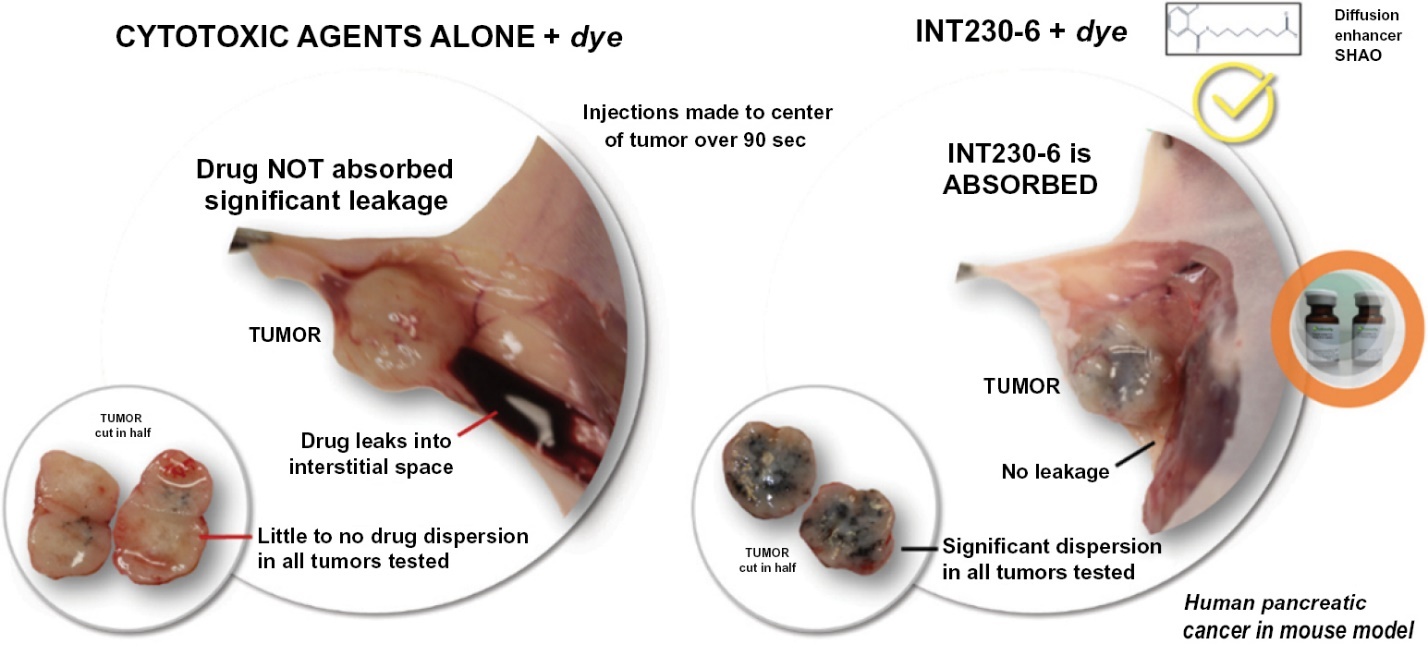
As part of our own research, we formulated cisplatin in water without the SHAO and added a noncolloidal dye. When injected into a human pancreatic tumor grown in a mouse model, we observed that the water formulation of the drug without the SHAO was not absorbed in the tumor. The liquid mostly leaked from the tumor. However, the formulation that
incorporated SHAO was readily and rapidly absorbed by the tumor in a dose dependent manner as shown in Figure 2 below.
Figure 2 – Comparison of drug dispersion/absorption in tumors with and without our DfuseRx technology.
Dense human pancreatic cancer BXPC-3 tumors were grown in severe combined immunodeficiency mice. Injections using a metered pump of the cisplatin with dye in water were compared to INT230-6 with dye. Fourteen mice were treated. INT230-6 is well absorbed and distributed throughout tumors (right side images) compared to the drug alone in water which leaks out (left side images). Data published in the International Journal of Molecular Sciences June 2020 doi.org/10.3390/ijms21124493.
In addition to formulation experiments we conducted growth inhibition experiments using large tumors (>300 mm3) and treated with low drug doses. Typically, research conducted by other companies developing cancer products use small tumors (25 to 100 mm3). Such companies also often use large drug doses in their studies with drug amounts that are five to fifty-fold above our dose amounts. Our product candidate can completely eradicate murine tumors, an effect that is termed a complete response (CR). Most competitors show only a slowing down of the tumor’s growth rate over time.
INT230-6 regresses tumors over time as shown in Figure 3 panel A and extends animal life compared to the drugs given alone intratumorally at the same dose without our technology. In addition, our drug candidate shows superior efficacy given intratumorally compared to dosing the drugs intravenously. Often animals with a CR are permanently protected against the cancer. This means upon re-inoculation with the same cancer new tumors do not grow. The protective effect happens whether the cancer cells are reinoculated under the skin or administered intravenously indicating a broad systemic immune protection.
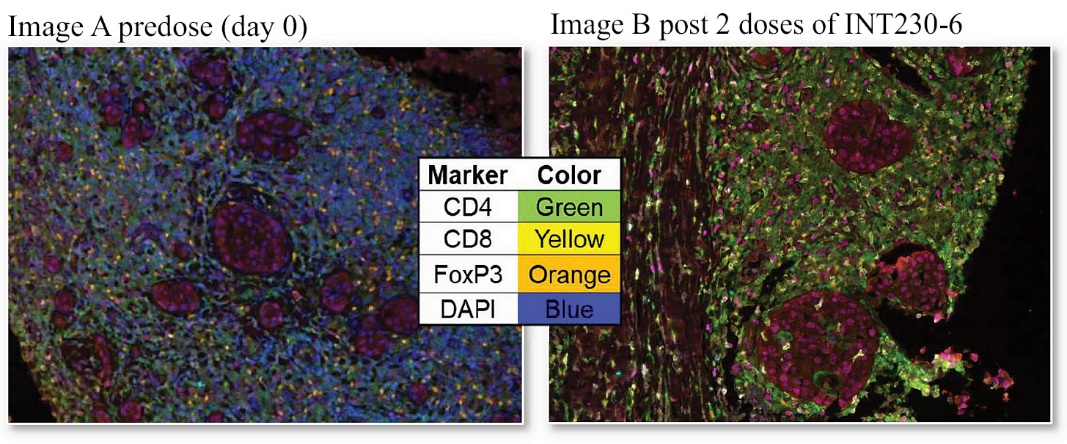
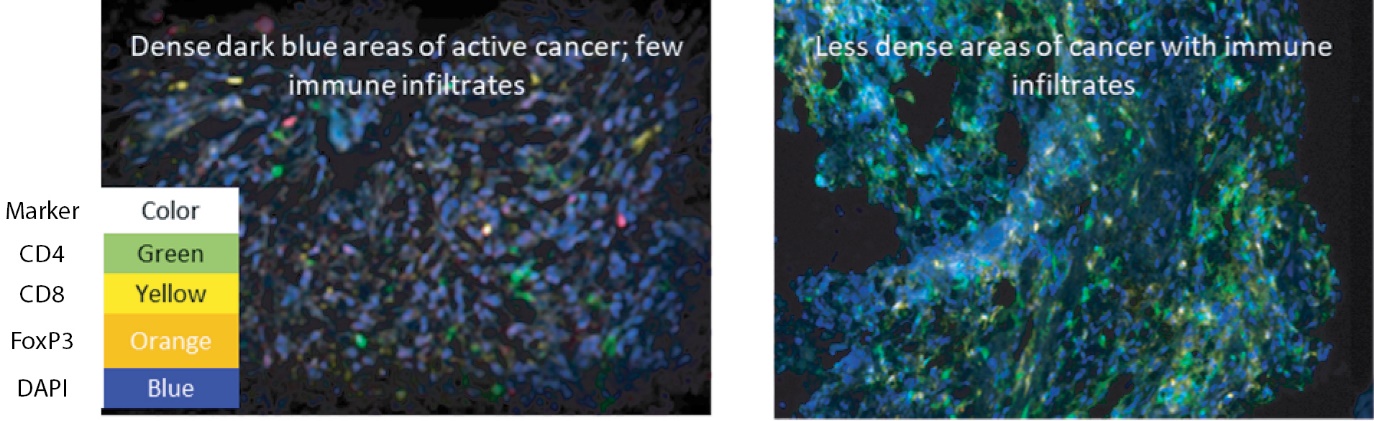
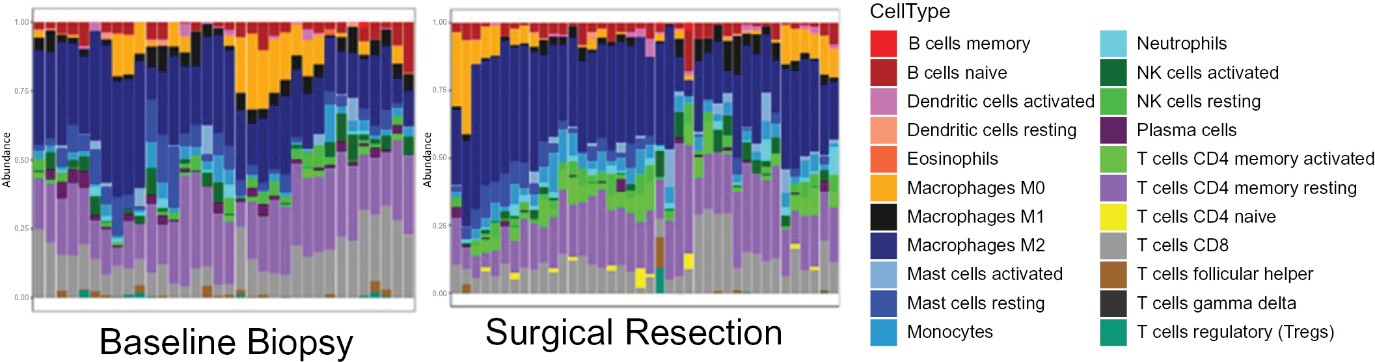
Through our research collaboration with the NCI, we generated data regarding the mechanism of action for our lead product candidate. INT230-6 shows direct tumor killing and immune cell activation. The direct tumor cell death is caused by action of the two potent agents (cisplatin and vinblastine sulfate). Data generated to date indicates infiltration of dendritic cells into the tumor which can present antigen to activate CD8 and CD4 immune T-cells against the cancer. Survival and tumor eradication are mostly driven by CD8+ T-cells. Thus, our product candidate generates high quality, vaccine-like antigen from the attenuated tumors to promote the immune activation. The Company also published data showing increases in dendritic cells, macrophage, T-cells and Natural Killer (NK) cells 10 days after intratumoral treatment in mouse colon tumors. Selective immune depletion of CD4 and CD8 abrogates the therapeutic effect. Figure 3 panel B that shows the influx of various immune cell into the tumor microenvironment.
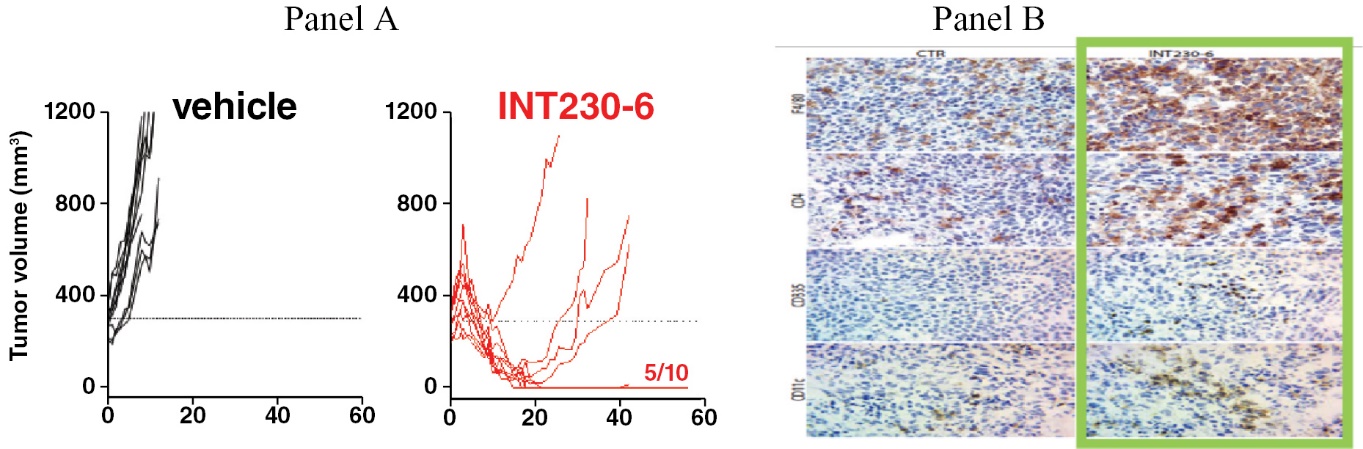
The scope of the NCI studies was to assess growth inhibition, survival and immune activation. Naïve mice were SC challenged with 1 × 106 C26 cells into the right flank. Vehicle or INT230-6 (0.5 mg/ml cisplatin, 0.1 mg/ml vinblastine sulfate, 10 mg/ml IT-006 cell penetration enhancer also referred to as SHAO) were intratumorally (IT) administered into 300 mm3 (approx. 8.5 mm in diameter, 100 μl/400 mm3 C26 tumor) SC tumors (n = 10/group) for 5 sequential days (day 0 to 4) and tumor growth was monitored. The fraction 5/10 indicates the number of complete responders. The log rank test indicates a significant difference between the groups (p<0.0001).
Figure 3 — Mouse data showing tumor reduction and immune activation
In Panel A on the left, 100% of animals receiving INT230-6 treatment for 5 days have a slight increase followed by a decrease from baseline, with 50% of animals having a complete response compared to no treatment controls with no decrease or complete responders (data generated by the NCI see OncoImmunology 2019 Vol 8 No 10; 15). Panel B cell staining shows an increase in the immune infiltrates. Data from Int. J. Mol. Sci. 2020, 21, 4493.
INT230-6 is Synergistic with Checkpoint Blockade
Nature has created checkpoints on the immune system to regulate the activity of the immune cells. These pathways are crucial for self-tolerance to prevent the immune system from attacking healthy cells indiscriminately. Large pharmaceutical companies such as Merck, Roche, AstraZeneca, Pfizer and BMS have developed new types of anti-cancer anti-body drugs with the ability to modify and block the checkpoints on the immune system.
Our results show strong benefit in regressing tumors with the combination of INT230-6 and checkpoint inhibitors which leads to improve survival. The data showed the combination of our product candidate with either anti-PD-1 or CTLA-4 antibodies in a dual tumor (metastatic) cancer mouse resulted in additive benefit. The data was generated by our partners at the National Cancer Institute and under our CRADA and published (OncoImmunology 2019 Vol 8 No 10; 15).
Preclinical Good Laboratory Practice (GLP) Safety of INT230-6
During a meeting in 2014 with the FDA we reached agreement on an accelerated safety and manufacturing program. We successfully completed the needed tasks to begin clinical testing that included conducting pharmacology studies (showing activity of the drug), toxicology studies in two animal species, analytical methods development, manufacturing scale up, and regulatory submissions. All these steps were completed by 2015. The data showed that the use of SHAO did not change or increase the toxicity of cisplatin or vinblastine sulfate. Analytical results showed the two drugs remain unchanged chemically when INT230-6 is stored properly, which is in a standard freezer at -20°C.
Clinical Regulatory Interactions
In the United States, the FDA regulates drug and device products under the Federal Food, Drug, and Cosmetic Act and its implementing regulations. We filed our IND application for our study IT-01 entitled “A Phase 1/2 Safety Study of Intratumorally Administered INT230-6 in Adult Subjects with Advanced Refractory Cancers” and held a meeting with senior FDA officials in November 2016. In December 2016, the FDA provided us a “Study May Proceed” letter.
We also met formally with the HC in a CTA meeting in 2016. We filed the CTA and held meetings with senior HC officials. Health Canada provided us a “No Objection” letter in early 2017. As we have progressed our study, we filed several amendments since 2017 and have received “No Objection Letters” each time from Health Canada. We have been treating patients continuously under both our IND and CTA since May 2017.
The regulatory agencies agreed to permit setting the drug dose based on tumor volumes rather than using alternatives such as dose based on a patient’s height and weight. Our belief is that using the patients’ total tumor burden (“TTB”) instead of body size is a more personalized and precise approach to ensure that patients receive an appropriate dose for their unique cancer burden. Better dosing could lead to maximized efficacy with minimized side effects. In our clinical trial, tumor volume is calculated from radiographic imaging on target tumors at baseline. Dose for a given tumor is set based on its size.
IT-01 Phase 1/2 Clinical Trial
Study IT-01, was completed in 2023. The study design permitted our product candidate to be tested in several different cancer patient populations with dosing into both superficial e.g. squamous cell, thyroid, breast, head and neck, lymph, skin, and deep body cancers such as those found in pancreatic, uterus, liver, kidneys, colon, bile duct, fat, muscles (sarcoma) and lung. The clinical trial sought to determine the safety and potential efficacy of dosing INT230-6 directly into several different types of cancers. We tested our product candidate in over 20 different cancer types.
IT-01, is listed on clinicaltrials.gov; NCT03058289. The hospitals that enrolled patients in the United States were: the Sydney Kimmel Cancer Center at Johns Hopkins, The Fox Chase Cancer Center at Temple University, the University of Southern California’s Norris Cancer Center, LA County and HOAG Presbyterian Hospitals of the University of Southern California medical system, the UMASS Memorial in Worchester Massachusetts, Columbia Presbyterian in New York, the Princess Margaret Hospital which is part of the University Health Network in Toronto, and the Houston Methodist.
In 2019 we partnered with Merck and in 2020 we partnered with BMS. We treated 30 patients having various cancers using INT30-6 with Merck’s Keytruda® (pembrolizumab) and 18 patients with BMS’s Yervoy® (ipilimumab). A schematic of the final Phase 2 study’s three dosing cohorts is shown in Figure 4 below. The final data and clinical study reports were provided to the partners in December 2023.
Figure 4 — Schema of the 3 final dosing groups for the metastatic study IT-01.
Results from IT-01 Phase 1/2 Clinical Trial
Safety
The Phase 1/2 study treated refractory patients, who failed multiple lines of therapy. One hundred ten (110) subjects were treated in IT-01. The results of the escalation portion, which included up to 175 mL per session every two weeks, indicated a favorable safety profile of INT230-6 with or without immunotherapy, with only 8 patients out of 64 on INT230-6 alone experiencing grade 3 related adverse events. The most frequent related adverse events include localized tumor related pain.
The majority of treatment related adverse events have been low grade (grade 1 or 2). A total of 15 patients out of 110 (13.6%) had at least one grade 3 adverse event in study IT-01. The primary grade 3 events have been pain, fatigue vomiting anemia, rash, dehydration and dizziness. There was one grade 4 adverse event, a decrease in the number of neutrophils, the most common type of white blood cell that contributes toward the healing of damaged tissues and resolving infections. There were no grade 5 treatment related adverse events reported. No maximum tolerated doses were established.
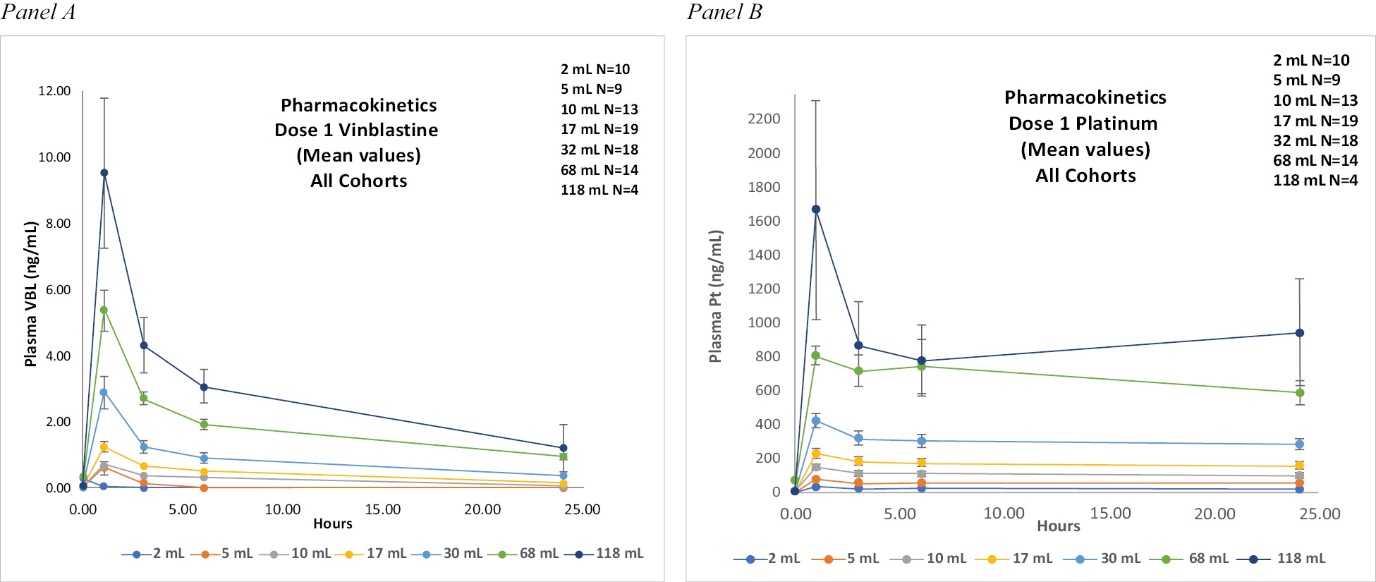
Even though our product candidate is dosed directly into the tumor, a key element of safety is to observe how much drug enters the bloodstream. Toxicities are linked to the circulating levels of the active agents in the blood. We measure circulating concentrations of the three main ingredients, SHAO, cisplatin (as platinum metal) and vinblastine sulfate, in the blood. This type of data is referred to as pharmacokinetics (“PK”). Data that measured the circulating levels of the key ingredients has been generated from the ongoing study in metastatic patients. The amount of vinblastine sulfate seen in
plasma of patients is much lower than a lesser dose given IV. Cisplatin is reduced to metal rapidly and is challenging to measure in blood even for IV dosing. A measurement of vinblastine sulfate provides a better understanding of the PK.
In our study, vinblastine plasma concentrations increased proportionally to the amount of drug administered. In essence, the concentration of vinblastine seen in the blood increased proportionally to the dose given intratumorally. See Figure 5 Panel A. This effect is independent of the cancer type and highly reproducible. As would be expected, the amount of the vinblastine seen in the plasma when given intratumorally was less than 5% of the blood concentrations had the drug been given intravenously. Our two highest average doses of INT230-6 were 118 mL and 80 mL. These dose volumes contain 11.8 and 8 mg of vinblastine sulfate and result in 9 and 6.8 nanograms per mL of vinblastine in blood plasma, respectively, at one hour post-dose.
At six hours post-dose, the amount dropped to about 3 and 2.2 nanograms per mL. Publications show the plasma concentration of a standard dose of vinblastine sulfate (6.5 mg for an average sized person) can be estimated. Based on pharmacokinetic studies of vinblastine in the literature (Links, M., Cancer Investigation Volume 17, 1999 – issue 7479-485), we estimated a vinblastine plasma level of 240 ng/mL at 6 hours for an IV dose of ~5.1 mg. Comparing our blood plasma concentration profile for vinblastine at various doses to the data from the Links cancer investigation indicates that >95% to 99% of the drug remained or degraded in the tumor post injection depending on the dose.
Cisplatin degraded rapidly. Measures of platinum metal are used in lieu of cisplatin for PK analysis as shown in Figure 5 Panel B. This drug retention in the tumor spares the patient the debilitating side effects of circulating drug. Indeed, the low observed plasma levels of the potent agents following INT230-6 dosing correlates with the low grade of side effects observed. Thus, IT dosing INT230-6 compares favorably to the toxicities normally associated with cisplatin and vinblastine sulfate when given intravenously at comparable doses.
Figure 5 — Free vinblastine (VIN) levels and platinum metal in blood plasma over time for intratumorally (IT) administered INT230-6.
Cytotoxic components in INT230-6 have minimal systemic exposure and short half-life. Most of the active drug remains in the tumor as a result INT230-6 appears to have favorable safety data to date.
RECIST (Response Evaluation Criteria in Solid Tumors) for Efficacy
A standard way to measure how well a cancer patient responds to a treatment is based on whether tumors shrink, stay the same, or get bigger. Efficacy assessments for evaluating changes in tumor size in clinical trials are typically conducted with standardized oncology response criteria, for example, Response Evaluation Criteria in Solid Tumors known as RECIST or a newer version 1.1 (RECIST 1.1). There are additional guidelines for immunotherapeutic trials (iRECIST). These criteria measure the change in longest diameter of tumors to assess drug response. An increase in longest diameter of > 20% is considered progressive disease. The rationale behind this is that tumors should generally become smaller. The main benefit of iRECIST is to afford physicians the opportunity to confirm progression with a follow up scan of the tumors 1 to 2 months later. However, both RECIST 1.1 and iRECIST criteria were designed only to assess response to systemic therapies.
Our study initially employed RECIST 1.1, and subsequently, iRECIST methods for determining the efficacy of INT230-6. INT230-6 induced tumor regression in both injected and non-injected lesions in several patients. However, tumors often increased in the longest diameter prior to shrinking using our drug, which we attribute to three factors. The first is high absorption by the tumor of our drug. Prior to the first efficacy scan, during the first two months (after 5 sessions) of INT230-6 treatment, patients would have received depending on the cohort a dose volume of drug injected into the tumor equivalent to 25% to 250% of the tumor’s volume. The second factor is an infiltration of immune cells into the tumor that can increase the longest diameter. Finally, tumors can become cystic. We have reported these data at major medical conferences (ASCO 2021, 2022, 2023, CTOS 2022, 2023) to indicate that RECIST methodology may be an inaccurate measure of clinical benefit for intratumoral INT230-6.
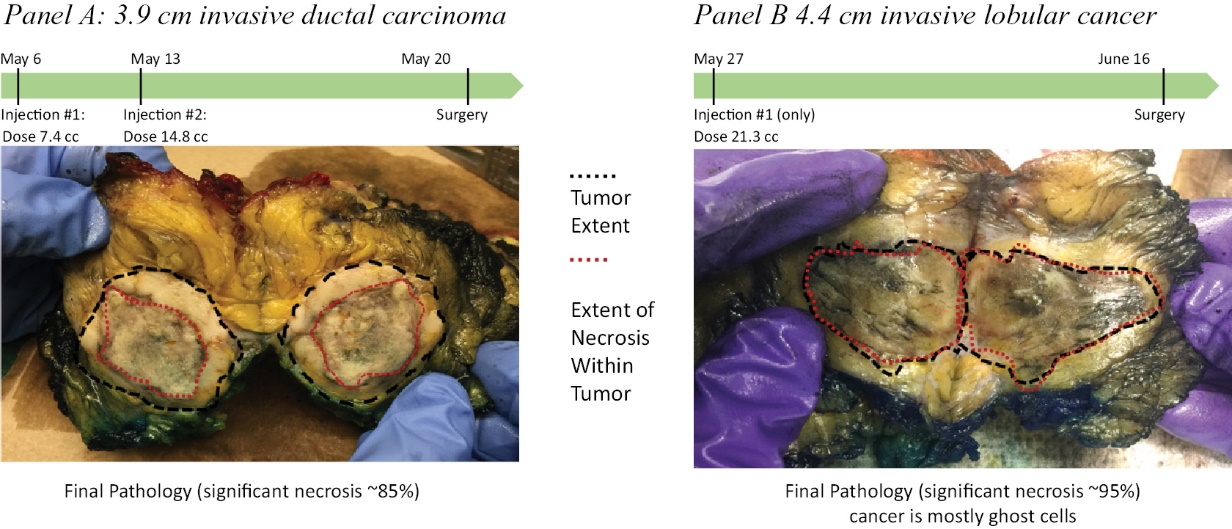
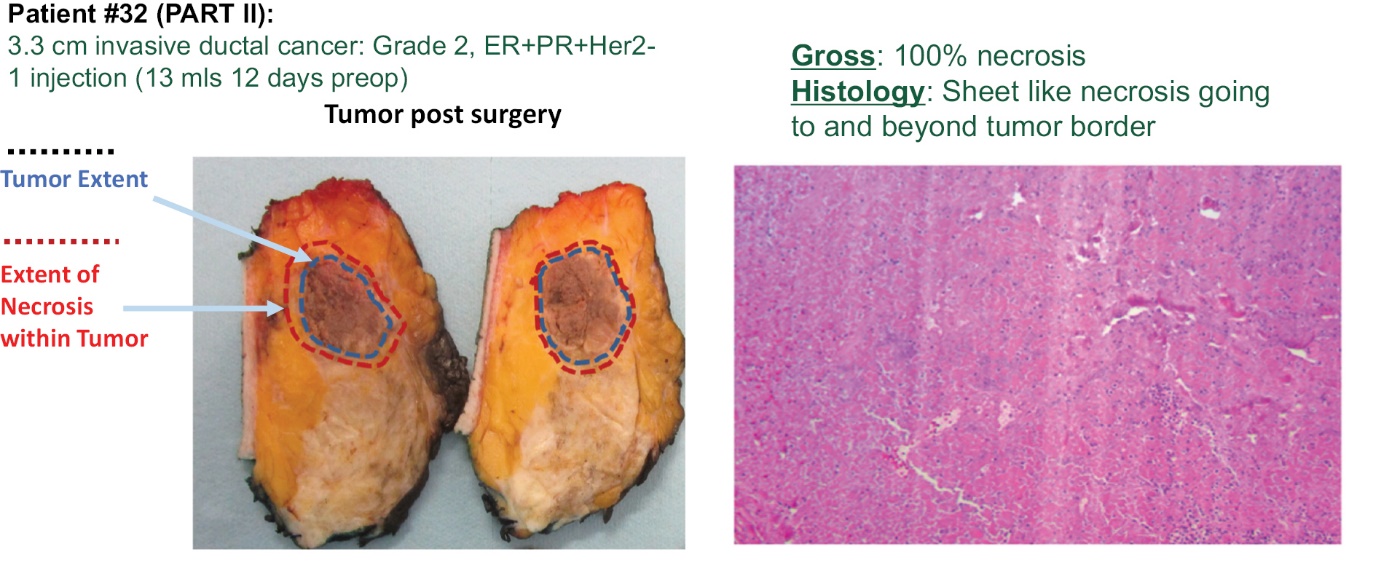
Tumor Death (Necrosis)
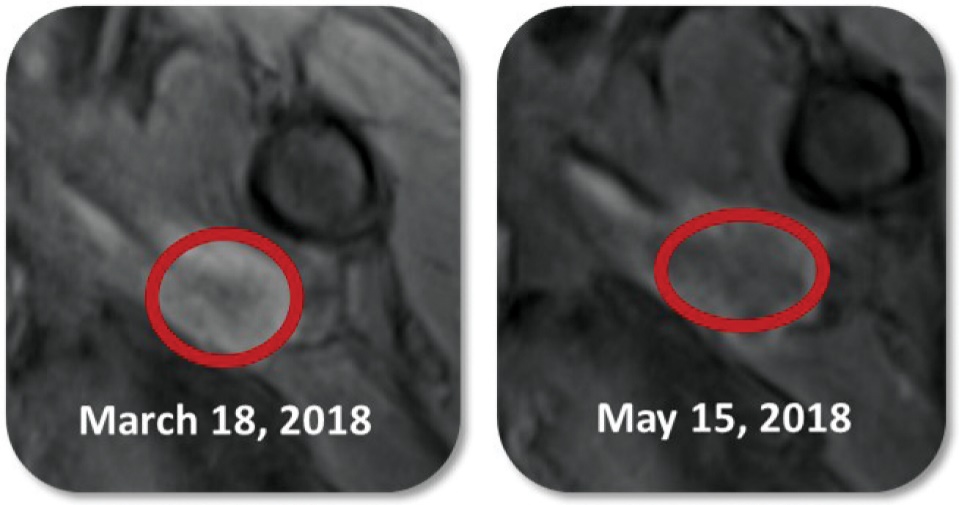
Investigators report significant necrosis (reduced contrast uptake in the CT image) in many injected tumors including adrenocortical, breast, chordoma, colon, head and neck (H&N), lung, sarcoma and squamous cell. Figure 6 below is an example of a squamous cell tumor that became necrotic by the 2-month scan. The darker contrast of the tumors indicated that significant necrosis of the tumor occurred following treatment.
Figure 6 — Images showing that INT230-6 induces tumor necrosis (death) in the injected tumors.
The patient in these images had a squamous cell carcinoma. His cancer continued to progress after 2 surgeries, radiation, and chemotherapy. The patient enrolled in our study in January 2018 with two 10 cm3 deep tumor nodules in his upper arm muscle. The hospital recommended total arm and shoulder amputation. This subject received 4 intratumoral injections equal to 100% of his 2 tumors’ volume. The drug was dosed at ratio of 1 mL per 4 cc of tumor. In the red circle in the left panel there is bright contrast indicating active cancer. At the first scan on May 15, 2018, there was an increase in tumor size, significant necrosis (lack of contrast) and inflammation observed (right panel). This patient has retained his arm and shoulder and is alive as of the last follow up visit in December 2022.
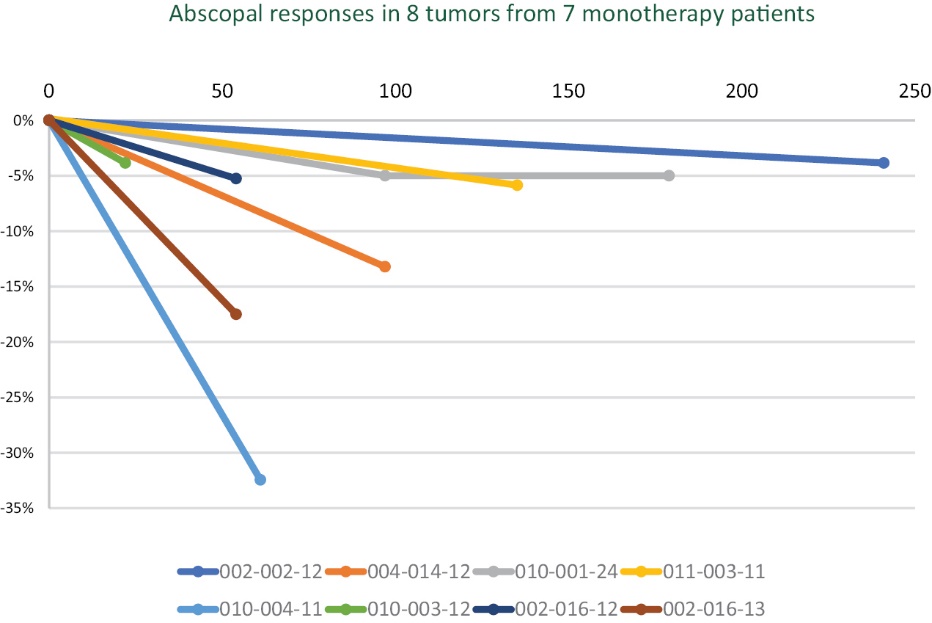
Abscopal Effects
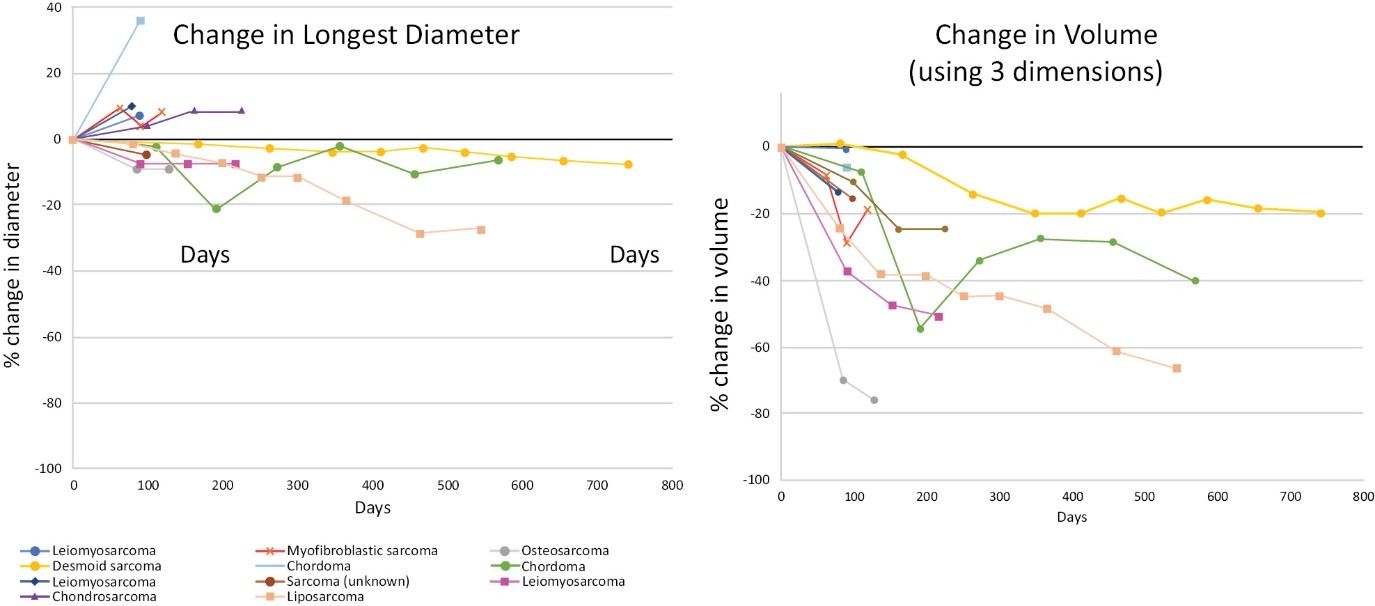
In the metastatic study several subjects showed tumor size reduction of non-injected lesions in lymph nodes, liver, lung, perineum, and retroperitoneal areas (i.e. abscopal effects to visceral lesions). The apparent abscopal effect was seen primarily in patients that received a dose greater than 40% of their TTB. Abscopal effect rates may be even higher than known. Sites did not report existing tumors under 1 cm in diameter. In addition, many tumors above 1 cm were not followed and unreported. We captured images from all subjects to be able to determine the true abscopal effect in all subjects at a future time and this analysis is in progress. Figure 7 below shows uninjected tumor diameter changes over time of patients with confirmed reports of abscopal effects.
Figure 7 — Change in longest diameter of uninjected tumors over time (abscopal effects) monotherapy subjects only.
Tumor Diameter and Corresponding Volume
For injected tumors, changes in longest diameter often do not correlate with changes in volume. Dosing is completed just prior to their first scan when the increase in tumor diameter is most likely to be highest. As noted above, RECIST measurements of whether a patient’s cancer is stable, decreasing or progressing are based on the changes in the tumor’s longest diameter. An increase in longest diameter above a threshold would indicate progression. In Figure 8, the graph on the left shows the change in individual tumors’ longest diameter over time. The graph on the right shows the same tumor’s volume over time. Tumors in many patients treated with INT230-6 can show an increase or no change in longest diameter with a decrease of the corresponding tumor’s volume. There is also a much greater volume decrease than expected for the slight decrease in longest diameter. In some cases, tumors can become cystic, which on imaging looks like a large increase. The increase in size was seen on scans until cystic tumors were drained. These data provide further evidence that RECIST may not be a good indication of efficacy for INT230-6.
Figure 8 — Chart showing that use of INT230-6 may increase tumor’s longest diameter while decreasing the tumor’s volume (sarcoma patients only).
In the left figure each color represents the change in diameters of an individual patient’s group of tumors. In the right figure the same color represents that same patient’s change in tumor volumes.
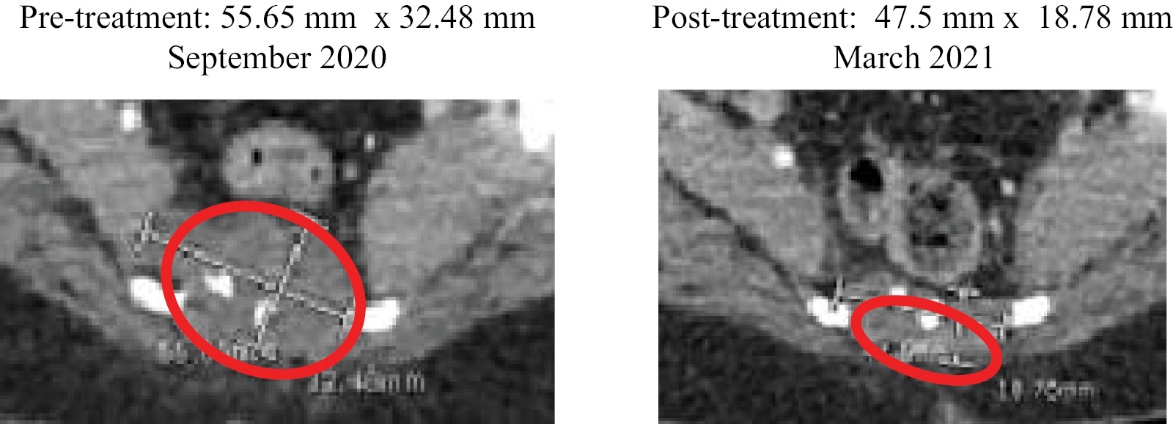
Visualizing a change in 3 dimensions also shows the limitations of using RECIST methods for determining efficacy for intratumoral INT230-6. Figure 9 shows the scan of a sarcoma patient’s tumor pre- and post-dosing. The longest
diameter declines by 15%, while the second longest diameter declines 42%. Using RECIST criteria, this patient would have been classified as having stable disease, whereas the World Health Organization, which uses the two longest diameters, would classify this patient as having had a partial response, which is a better outcome.
Figure 9 — Scan showing change in two longest diameters of an injected sarcoma tumor at the base of the spine.
We believe that RECIST measurements (longest diameter) are inappropriate to capture efficacy with INT230-6. As a result, overall survival, the FDA’s gold standard efficacy endpoint, is a better measure of INT230-6’s performance in metastatic cancer.
Disease Control Rate
Even though RECIST was deemed to be less accurate for measuring efficacy a secondary objective of IT-01 was to assess the preliminary efficacy of INT230-6 by measuring the disease control rate (DCR) based on the (RECIST) and immune RECIST (iRECIST) criteria. The DCR rates for the study are shown below.
| | | | | | | | |
Monotherapy cohorts | | Disease Control Ratea |
Total (all subjects) (N = 64), n (%) | | 75.0 (62.6, 85.0; 48) |
Dosed ≥ 40% TTB (N = 48), n (%) | | 83.3 (69.8, 92.5; 40) |
Dosed < 40% TTB (N = 16), n (%) | | 50.0 (24.7, 75.3; 8) |
| | | |
Sarcoma only (N = 15), n (%) | | 93.3 (68.1, 99.8; 14) |
Dosed ≥ 40% TTB (N = 11), n (%) | | 90.9 (58.7, 99.8; 10) |
Dosed < 40% TTB (N = 4), n (%) | | 100 (39.8, 100; 4) |
Prior treatmentb | | |
Yes (N = 31), n (%) | | 74.2 (55.4, 88.1; 23) |
No (N = 33), n (%) | | 75.8 (57.7, 88.9; 25) |
Survival — Phase 1 Basket Studies
The primary objectives of Phase 1 trials are to define the safety or toxicity profile of a new drug and to determine the dose for further evaluation in Phase 2 trials. Patients enrolled in Phase 1 are therefore placed at risk of toxicity, in exchange for an undefined and limited clinical benefit. Furthermore, patients who are considered for Phase 1 trials may be regarded as vulnerable because their physical condition may be deteriorating due to advanced cancer malignancy for which no further standard treatment options exist. Efficacy is not usually the primary objective. Most patients in Phase 1 studies have low survival expectations that ranges from 3 to 8 months depending on the type of cancer and patient’s incoming health. (see Chau, N., BMC Cancer volume 11, Article number: 426 2011).
Over the past two decades the development of a prognostic score to predict survival of patients treated in Phase 1 studies has been completed and validated by the Royal Marsden Hospital (RMH) in the United Kingdom. The score, which ranges from 0 to 3, is highly correlated of overall survival (OS) outcomes. A score of 0 suggests longest potential survival and a 3 worst. Many studies show that subjects enrolled in Phase 1 have survival of under 6 months when RMH scores greater than or equal to 1.
In our study IT-01 patients were enrolled whose cancer progressed following treatment using all approved and some experimental therapies suitable for their specific disease. Forty-three (43%) of patients had previous had an IV form of a platinum-based drug including cisplatin. Forty-four percent (44%) had previously received an anti-PD-1 antibody. Efficacy data from 64 patients enrolled in IT-01 is available from patients receiving INT230-6 alone (referred to as monotherapy). There were over 820 different tumor injections conducted over the course of the trial with over 502 being into visceral deep tumors.
Study IT-01 was a Phase 1/2 dose escalation (i.e. the Phase 1 basket portion) and Phase 2 (expansion of specified cancer types). These types of studies are primarily testing safety in the Phase 1 and determining whether there is an efficacy signal in the expansion compared to historical data. There was no control arm in IT-01 and no randomization. Therefore, there is no comparator to determine the significance of any given endpoint. See Table below for the patient population.
Final INT230-6 monotherapy population (n=64) in IT-01
| | | | | | | | | | | | | | |
Type of Cancer | | Monotherapy
Number of
Patients | | Monotherapy
Percent of
Population |
Sarcoma | | 15 | | 23% |
10 Othera <4% each | | 13 | | 20% |
Skin (Melanoma/Merkle) | | 6 | | 9% |
Squamous cell carcinoma | | 6 | | 9% |
Colorectal | | 5 | | 8% |
Breast | | 4 | | 6% |
Head and neck | | 4 | | 6% |
Ovarian | | 4 | | 6% |
Pancreatic | | 4 | | 6% |
Cholangiocarcinoma | | 3 | | 5% |
____________
aOthers include: Adrenocortical carcinoma, Anal, Bladder, Cervical, Eccrine, Lung, Metastatic Cancer of Unknown Primary, Pseudomyxoma peritonei, Renal, and Thyroid.
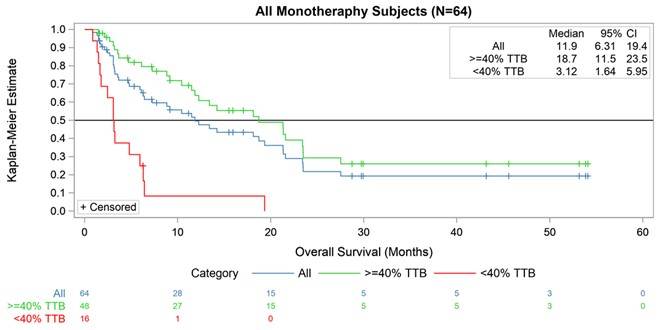
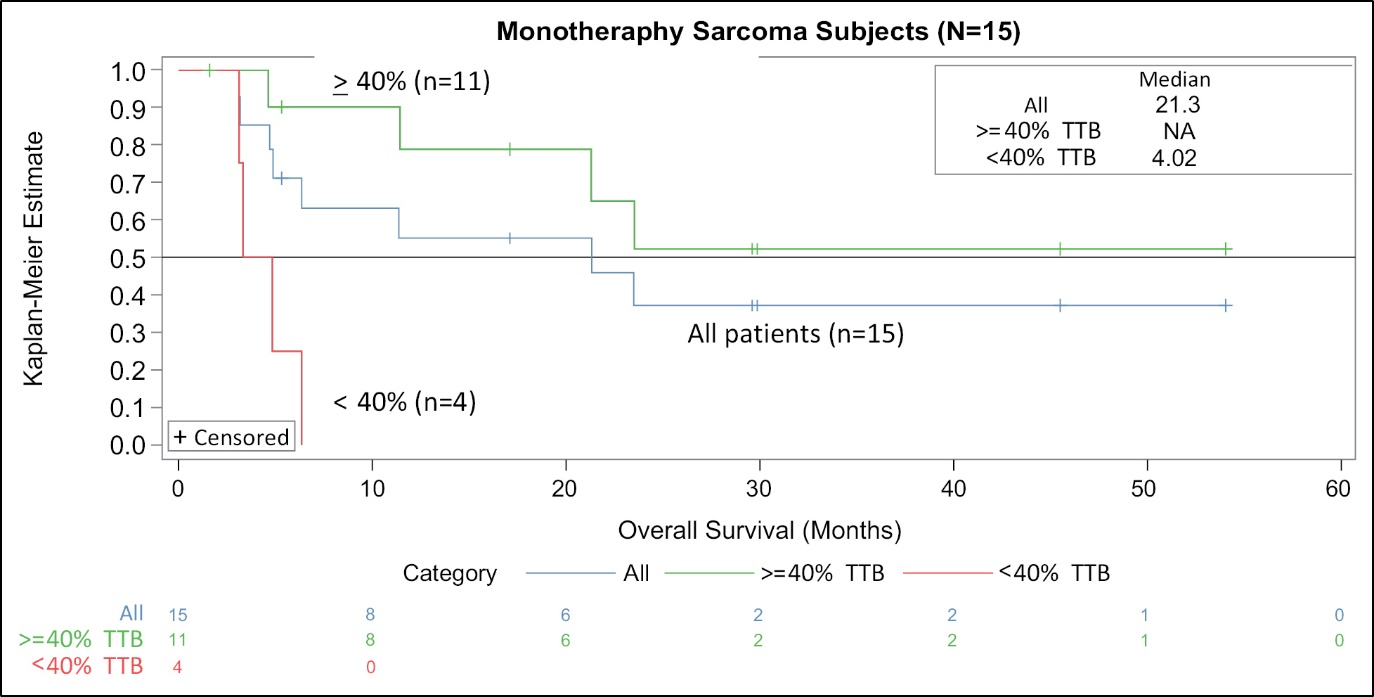
Patients receiving a monotherapy dose of INT230-6 above 40 percent of their TTB measured in cubic centimeters had a statistically significantly longer survival than patients who received treatment to less than 40% of their TTB. The subjects receiving a dose >40% of their total incoming tumor burden also lived much longer than would be expected for patients in a Phase 1/2 basket study. These data indicate a potentially active drug. Given the small size of the population, the heterogeneity of the cancers and variability of the incoming tumor burdens, the high and low dose groups may have been different in a way that we may not have been able to measure. We observed a strong overall survival signal in just sarcoma patients; however, this Phase 2 population size was also too small to properly assess effectiveness of INT230-6. As a result, we have determined that overall survival, an endpoint preferred by the FDA for cancer clinical trials, is the most appropriate metric to prove efficacy of our drug candidate to treat metastatic disease. Study IT-01 also indicates that soft tissue sarcoma, at cancer type with high unmet medical need, would be a suitable disease for a Phase 3 trial to evaluate the efficacy of INT230-6.
In our metastatic study survival appears to be impacted by the total dose a patient received relative to number and size of their tumors. Patients receiving a higher percentage of drug (mL) relative to their TTB (cm3) remained on study longer regardless of the cancer type. A patient’s TTB is calculated by adding up the volumes of all reported tumors. Simply stated, the more drug given to more tumors, the more likely a subject would be alive longer, though not all tumors need be treated. Killing more of a patient’s cancer is beneficial.
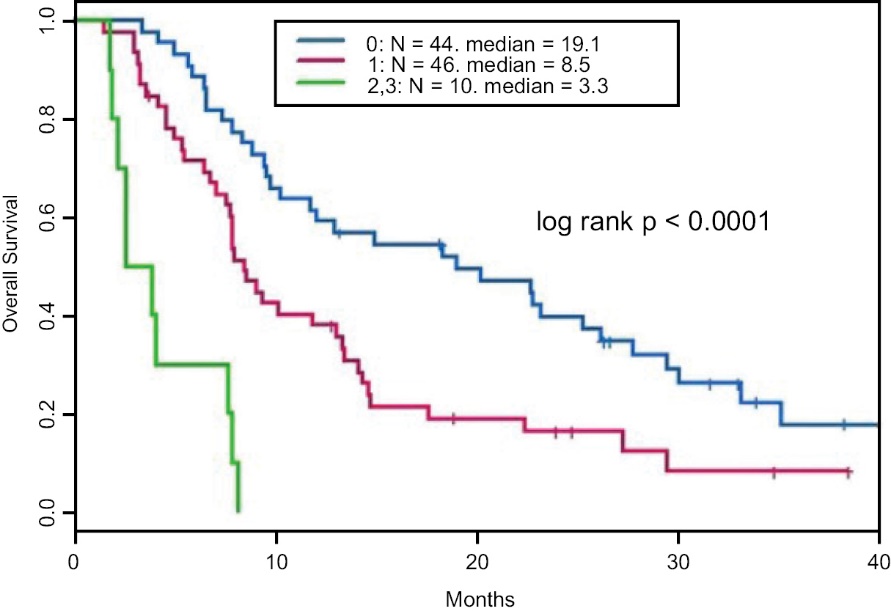
The probability of survival for a given population can be plotted. Figure 10 panel A below illustrates the survival for all monotherapy INT230-6 subjects. See Table below for the patient population.
Treating only with our drug candidate, approximately 50% of patients would be expected to be alive at one year (blue curve) with a median overall survival (mOS) expected of 11.9 months. Subjects dosed an amount of INT230-6 that was less than 40% of their TTB had a mOS of 3.1 months. This result is shown in the red curve and is comparable to survival expected in historical Phase 1 basket studies (See Chau, N., BMC Cancer volume 11, Article number: 426 2011). Patients that received a dose of INT230-6 to greater than 40% of their TTB had a ~63% chance of being alive at 1 year and the
median overall survival was 18.7 months. These results indicate that survival improves for those dosed to greater than 40% of their TTB compared to those receiving under 40%. While there were no differences statistically in the two populations with regards to incoming tumor burden; the sample size is small and the average values for the green curve was lower.
Figure 10 — Kaplan Meier Estimates of Sarcoma Patient-Survival Dosing INT230-6 in study IT-01
Exploratory analysis of dose relative to TTB was conducted. Many tumors, including all under 1 cm in diameter, were not reported and so TTB is likely underestimated.
Biomarker Analysis
A cancer cell’s surface expresses a unique set of proteins specific to the patient and their cancer type. Certain immune cells can “read” the cell surface to create a patient-specific immune response. However, as noted above, live cancer cells can send signals that can block the immune cells from entering the tumor. There is a constant “cat and mouse” battle between the cancer cell and the immune system.
Other local treatments such as radiation or ablation destroy the cell surface. Our technology disperses potent killing agents throughout tumors and enables the potent killing agents to diffuse into the cancer cell without damage to the cell membrane. When the tumor’s cancer cells are no longer alive, the ability of the immune system to identify the cancer and mount a response can be increased.
We collect tumor tissue before and after dosing of our drug candidate from patients injected tumors. We analyze for live and dead cancer cells (referred to as necrotic cells). Our data shows that our drug candidate can kill cancer cells over a couple of weeks and activate an immune response. We have observed these effects in multiple cancers.
Methods used
INT230-6 injections were conducted on the first treatment cycle’s first day (referred to as C1D0) and on the fourteenth day (C1D14). Pre and post-dose biopsies from the same injected tumor were obtained on C1D0 and again 28 days later just prior to the 3rd dose on the first day of the second treatment cycle (C2D0). To determine the percentage of viable tumor cells and necrotic (dead) cancer cells pre and post two treatments, we conducted analysis on the collected tissue following haemotoxylin and eosin (“H&E”) staining. H&E tissue analysis helps identify different types of cells and provides important information about the pattern, shape, and structure of cells in a tissue sample.
For many patients, we observed substantial reductions of cancer following the two injections of INT230-6 alone. Below are data on cell killing and immune activation from the two cancer types, breast cancer and sarcoma, for which we are planning Phase 3 programs. We also use immunohistochemistry (IHC) staining to help assess cancer and various immune cell populations, as well as the degree of cancer cell proliferation in the treated tumors.
Necrosis Results in Breast Cancer
Figure 11 shows tissue taken from a breast cancer patient. The pre-dose (C1D0) samples stained positive (dark purple) indicating significant amounts of cancer throughout the sample. However, 28 days later (C2D0), there was almost no cancer observed in the collected tissue. Magnification is 400µ.
Figure 11 — Images from match pair biopsied tissue samples pre and post two INT230-6 injections:
Immune Response in Breast Cancer
In preclinical studies, our technology reduces the cancer levels substantially and causes the influx of immune cells into the tumor. The below images from a breast cancer patient confirm that this effect also occurs in humans. Applying a special set of stains to the biopsied tissue enables the measurement of immune cells inside the tumor. We observe infiltrating immune cells in the tumor. In Figure 12 (below) the first panel (Image A) there is extensive cancer as the blue color seen is for 4´,6-diamidino-2-phenylindole, a blue-fluorescent DNA stain (DAPI) and the marker of live and proliferating cancer. The green and yellow colored cells are immune cells. The second panel (Image B) shows that at 28 days after the first dose there is a markedly reduced amount of live cancer (less blue stain). In addition, the green/yellow stained cells, representing CD4 and CD8 T-cells, are increased dramatically throughout the entire tissue.
Figure 12 — IHC Staining of breast cancer tissue for immune cell infiltration pre and post dosing of INT230-6
Necrosis Results in Sarcoma
Several patients with metastatic soft tissue sarcomas have been enrolled in the study. As was seen with breast cancer and multiple other tumor types, there were substantial reductions of cancer in the biopsies pre and post in sarcoma patients. As shown in Figure 13. Image A is the stained tissue sample (pre-dose) that shows significant cancer (dark purple cells) throughout the tissue sample. Image B is the stained tissue sample taken on day 28 after two doses of INT230-6 (day 0 and day 14) that shows significant reduction in the cancer (Magnification 3.7x).
Figure 13 — Images from match pair biopsied soft tissue sarcoma subject 010-001 pre and post two INT230-6 injections
Immune Response in Sarcoma
We also measured DAPI and activated T-cells from a sarcoma tumor. The results again confirm that for this non-immunogenic tumor type, there is also a substantial reduction of tumor cells as seen by the decrease in the marker post INT230-6 treatment. Figure 14 shows the influx for sarcoma patients into the tumor of CD4 and CD8 T-cells at 28 days following the first dose.
Figure 14 — Staining of biopsied sarcoma tumor tissue pre and post dosing of INT230-6
| | | | | | | | | | | |
| | Image A - Predose | Image B - Post dose |
Results of the H&E analysis as well as the multiplex IHC staining show substantial cancer cell reduction, decreases in proliferation, and increased immune infiltration after INT230-6 dosing. The totality of the data indicate the drug has the ability to kill cancer and increase the immune response in multiple sarcoma types.
INT230-6 Efficacy in Soft Tissue Sarcoma
Sarcomas are a rare and heterogeneous group of solid tumors derived from mesenchymal cell origin. Although single agent or combination anthracycline-based chemotherapy provides some benefit for the treatment of advanced sarcomas, prognosis is still unfavorable with median overall survival of 12 – 16 months and there is significant unmet medical need. By the time subjects fail approved therapies and enter Phase 1 studies patients’ median overall survival is typically 3 – 8 months (see Subbiah, V Scientific Reports | 6:35448 | DOI: 10.1038/srep35448) depending on certain risk factors such those found in the RMHI score.
Thirty patients with sarcoma were treated in study IT-01. Fifteen received INT230-6 monotherapy and 15 received INT230-6 with immunotherapy. Enrolled subjects receiving INT230-6 had a median of 3 (0, 8) prior therapies, median age
of 64 and 13% were ECOG 0, 80% ECOG 1. Those receiving the combination with ipilimumab had a median of 4 (0, 9) prior therapies, median age of 64 and 38% were ECOG 0, with 62% ECOG 1.
The sarcoma types were Leiomyosarcoma, Liposarcoma, pleomorphic sarcomas, chondrosarcoma, chordoma, spindle cell sarcoma, fibrosarcoma, osteosarcoma, myofibroblastic sarcoma, desmoid type, and Kaposi sarcoma. The INT230-6 dose delivered at a single visit was up to 242 mL (112 mg of cisplatin, 24.2 mg of vinblastine sulfate) into one or more tumors. The VIN given exceeded the typical 5.1 mg starting IV dose for an average size person. The CIS given was equivalent to a typical IV dose. Safety in sarcoma population remained favorable. The most common treatment-related adverse events (TRAEs) in evaluable monotherapy subjects were localized pain, fatigue, decreased appetite, nausea, most of which were low grade. Please see Table 1 in the “Results from IT-01 Phase 1/2 Clinical Trial” portion of our “Business” section on page 67 of this report for more information.
We compared our Phase 1/2 basket study survival data in soft tissue sarcoma (“STS”) to overall survival data generated from three published clinical Phase 1/2 basket trials in sarcoma. In our trial, IT-01, fifteen (15) STS patients received only INT230-6 monotherapy and 11 have received the combination with ipilimumab. The 3 studies used were:
•Jones Cancer Chemother Pharmacol (2011) 68:423 – 429, Clinical benefit of early Phase clinical trial participation for advanced sarcoma patients.
•Cassier et. al., Annals of Oncology 25: 1222 – 1228, 2014 Outcome of patients with sarcoma and other mesenchymal tumours participating in Phase I trials: a subset analysis of a European Phase I database.
•Subbiah et. al., Scientific Reports | 6:35448 2016, Evaluation of Novel Targeted Therapies in Aggressive Biology Sarcoma Patients after progression from US FDA approved Therapies.
Each of these publications report use of the Royal Marsden Hospital index (RMHI). As noted above the RMHI is validated score predictive of overall survival for cancer patients in basket studies. A subject obtains 1 point depending on their number of metastatic sites, pre-dose plasma lactase dehydrogenase level and albumin concentrations. Each of the 3 studies report the median overall survival results for subjects for various RMHI values as shown in the table below.
Median OS in Phase 1 Basket studies
| | | | | | | | | | | | | | | | | | | | |
Study | | Jones | | Cassier | | Subbiah |
| Median OS | | 7.6 months | | 9.1 months | | 9.6 months |
| | CI (4.8 – 10.4) | | CI (6.3 – 11.8) | | (CI (8.1 – 14.2)* |
____________
*44% of Subbiah study subjects had a RMHI score of 0 versus 26% in Sponsor’s study IT-01
Though the sarcoma subtype mix between the 3 literature study populations is not matched exactly, mOS from the Jones, Cassier and Subbiah studies are similar. Subbiah reports the overall survival of sarcoma patients for a given RMHI score (Figure 15 below) from the publication shows the strong correlation between RMHI score and overall survival.
Figure 15 — Overall Survival based on RMHI scores in soft tissue sarcoma (Subbiah data)
We were able to estimate the RMHI score in our study for each patient receiving only INT230-6. Subjects in our study primarily had a RMHI score of 1 (33%) or 2 (40%). To be more confident that we are matching the population when comparing our drug to the Subbiah population, we created a synthetic Kaplan-Meier (KM) control curve from the Subbiah dataset that approximated our patients’ RMHI score distribution (Figure 16 below). We chose Subbiah as the dataset, because it was the study that reported the longest survival of the three Sarcoma studies, and would be the most conservative data to serve as the basis for a synthetic control.
Our drug is dosed based on a patient’s total measured volumetric tumor burden. From our mouse studies we found that a dose volume to tumor volume ratio of 1 to 4 could nearly saturate a tumor. The average TTB of patients entering the trial was 200cc. However, when our study was initiated, the dose was limited to 5 mL per month to establish the maximum tolerated dose and generate safety data. This early dose to tumor ratio proved to be inadequate to treat most tumors. As a result, most early patients were under treated and the survival seen early in the study was typical of survival rates normally seen in Phase 1 basket trials. As we moved through the dose escalation Phase of our trial and generated favorable safety data, the dose amount frequency and loading into the tumor was escalated.
We observed that if a cumulative drug dose volume equal to 40% or more of the total reported presenting tumor burden was administered over 5 sessions every two weeks with maintenance, patients showed evidence of clinical efficacy as overall survival increased. For the INT230-6 monotherapy STS patients (N=15) with a median of 3 prior lines of therapy our median OS was 21.4 months with a confidence interval of 6.4 to 42.0 months (this dataset includes those subjects dosed with considerably less than 40% of their TTB). In those subjects receiving a dose greater than or equal to 40% of their TTB (N=11), the OS was not reached with ~400 days of median follow-up. Subjects receiving less than 40% of their TTB had a median survival of 4.0 months.
We calculated the KM synthetic control derived from the Subbiah basket trial matched to study IT-01 sarcoma population’s RMHI scores for all INT230-6 monotherapy patients that predicted our sarcoma patients should have had a median survival of 6.7 months.
Figure 16 — Survival of INT230-6 monotherapy sarcoma patients
Estimates of sarcoma subject survival using INT230-6 based on dose per TTB from study IT-01 compared to a synthetic control are shown in the table below.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | INT230-6 Dosed
<40% of TTB
(months) | | Synthetic Control
of sarcoma
patients
(2 prior lines)
(months) | | INT230-6 all
subjects
(months) | | INT230-6
Dosed >40% of TTB
Months |
Median overall survival, CI | | 4.0 | | 6.7 | | 21.3 | | Not reached with 400 days of median follow-up ( |
IT-02, The INVINCIBLE-2 Study in Presurgical Breast Cancer
In March 2021, we began a Phase 2 Randomized, Window of Opportunity trial evaluating clinical and biological effects of intratumoral INT230-6 against no treatment (the SOC) in early stage breast cancer patients awaiting surgery. The study completed enrollment and the database was locked in November 2023. The key efficacy endpoints were to (i) compare necrosis levels in tumors based on size and dose compared to saline control, (ii) the percentage of subjects having a greater than 50% reduction of viable cancer cells in their tumor compared to control, and (iii) the percentage of subjects who achieve a cell cycle arrest, defined as a reduction in the proportion of cells staining positive for Ki67, a widely used marker of cancer cell proliferation for systemic therapy. According to our estimates using the National Cancer Database (NCBD), approximately 40% of patients diagnosed with breast cancer annually, there are nearly 100,000 that have no therapeutic treatment following diagnosis. Women undergoing surgery typically wait approximately 2 to 6 weeks to have the procedure.
The trial was a two-part Phase 2, randomized, open label, multi-center study that has completed enrollment of 91 patients with early-stage breast cancer. In part 1, twenty-nine patients were randomized 2:1 to treatment or no treatment. Those in treatment received either up to three doses of INT230-6 on days 1, 8 and 15 post diagnosis or no treatment, the current SOC prior to resection. Part 2 of the study randomized patients 2:1 to one intratumoral injection of either INT230-6 or saline solution. IT-02 was conducted under the direction and supervision of Principal Investigator, Dr. Angel Arnout. The Ottawa Hospital conducted all subject enrollment, treatment and pathology for necrosis. The Ontario Institute of Cancer Research analyzed subject immune responses, Ki67 and conducted immune biomarker analysis. Ozmosis Research Inc., a Toronto-based CRO, managed the data and study in Canada. Intensity funded the trial and provided INT230-6 supply. There were no milestone payments, royalties or other compensation to be paid to any party. The agreement provided that each party will solely own any inventions generated in the clinical trial that relate solely to intellectual property owned by that party.
In study IT-02, the treatment group had a highly statistically significant increase in necrosis (tumor death) compared to the saline control group of 19% for the treatment group versus 1.3% for the saline control group (p=0.0002). For tumors with diameter of 2 cm or higher in longest diameter the treatment group had an average of 24% necrosis in 42 subjects vs.
0.8% for the saline control group in 8 subjects (p=0.0007) . In the study nine (9) subjects in INT230-6 treatment groups had a major pathological response (MPR) with a mean of 79.4% tumor necrosis. MPR is defined as having less than or equal to 50% residual cancer in the tumor (i.e. ≥50% of the tumor became necrotic). In the control groups, no subjects achieved an MPR (n=29).
IT-02 (The INVINCIBLE-2 Study) Tumor Necrosis via Diffusion
Tissue taken via biopsy from tumor in our metastatic study IT-01 shows that viable cancer cells are significantly reduced. However, in our INVINCIBLE 2 study, surgeons also removed the entire breast cancer tumor following INT230-6 injection. At the San Antonio Breast Cancer Symposium (“SABCS”) in December 2023 images showed that up to >95% of an entire large tumor greater than 4.3 cm can be killed on a single INT230-6 injection at the proper dose (in milliliters) relative to the size of the size of the tumor (in centimeters).
This result is seen in Figure 17 panel A and B. An ER+PR+HER2+ 3.9 cm grade 3 invasive ductal breast cancer tumor was treated on day 1 with 7.4 cc of INT230-6. Seven days later with another 14.8 cc. The tumor was then resected another seven days later. In panel B, a ER+PR+Her2- 4.4 cm diameter invasive lobular breast cancer tumor was treated with one dose of 21.3 mL of INT230-6, then resected 20 days later. The INT230-6 was able to kill 85% of the ductal tumor from Panel A. However, in the second panel, the drug was able to diffuse throughout nearly the entire tumor. The boundary of the tumor is shown by the black dotted lines and the red dotted lines show the extent of the necrosis. Pathology conducted on the excised tumor showed that there was only a small percentage of viable cancer cells in one area of the 4.4 cm tumor after a single dose of INT230-6 of 21.4 mL. More than 95% of the tumor was necrotic (dead) or ghost cells (cells without nucleus). These images show that diffusion distance is proportional to the amount given on a single dose. In panel C we show high necrosis after surgery of a subject with a 3.3 invasive ductal cancer, who received one INT230-6 dose of 13.3 mL. This patient’s tumor was characterized as having sheet-like necrosis to and just beyond the tumor edge.
Figure 17 Panels A and B — Showing the extent of the entire tumor and the area of dead cancer for various doses of drug; greater than 95% of the total tumor volume was killed by a single dose injections of INT230-6.
Figure 17 Panels C and D — 100% necrosis with correspond H&E staining
In the above figure the entire breast tumor has been removed. The black or blue dotted line shows the extent of the tumor, and red dotted line shows the extent of the necrotic (dead cancer) after treatment with INT230-6. For a given tumor diffusion distance and thus tumor killing is proportional to the amount of drug dosed. Both tumors shown with high grade (3) proliferative tumors.
IT-02 (The INVINCIBLE-2 Study) Immune Activation
Intensity presented positive INT230-6 data in patients with early-stage breast cancer in a podium poster spotlight discussion at the 2023 SABCS on December 8, 2023. INT230-6 demonstrated a systemic increase in the median diversity of T-cell repertoire in patients’ blood compared to baseline that was also much larger than a control saline injection and again showed that a single injection of INT230-6 can induce up to >95% necrosis of a tumor. INT230-6 demonstrated an increase in CD4 T-cells and NK cells within tumors and gene expression profiling revealed a treatment effect of up-regulation of immune pathways expressed by T-cell activation, lymphocyte activation and inflammatory responses. INT230-6 demonstrated a favorable safety profile and was well tolerated and patient interest in the new treatment was high. There was a reduced in Ki67 for the drug and control. Live cancer cells are needed to evaluate Ki67. Several subjects’ tumors receiving INT230-6 had such high necrotic percentages that measurement of Ki67 was not possible. Those subject’s samples were therefore noted as unevaluable. This result biased the Ki67 outcome against the drug and indicated that Ki67 was an inappropriate marker for INT230-6.
Enrollment in the study was rapid. We believe patients are highly interested in a product that can potentially destroy the majority of their tumor rapidly while waiting for their surgery and with the possibility to induce a systemic anti-cancer immune response. Surgery proceeded on time or without difficulty by the INT230-6 IT treatment. Adverse events are minimal — mainly transient, low-grade pain at the injection site.
An analysis of differential gene expression comparing pre-and post-treated tumor tissue samples in the control group compared to the drug treated group showed that over 200 more immune related genes were activated pre- and post-treatment compared to the controls.
As shown in Figure 18 below, within the tumor there was a relative increase in abundance of CD4 T naïve (light green) and NK cells post treatment (darker green).
Figure 18 — Relative abundance levels of immune cells present in the breast cancer tumor compared to current standard of care (no treatment controls.
Each bar demonstrates the immune cell abundance in a specific patient separated by cancer subtype, the left panel is the baseline cell population and the right panel is the post INT230-6 treatment. There was a relative increase in abundance of CD4 T naïve (light green) and NK cells (darker green) in the majority of patients post treatment.
In addition, INT230-6 demonstrated a systemic increase in the median diversity of the T-cell repertoire in patients’ blood compared to baseline that was much larger than a control saline injection as seen in Figure 19. The adaptive immune system is one of the body’s most powerful defenses. By being able to adapt, the body’s immune cells can be trained to attack undesirable cells or viruses anywhere in the body. T-cells are an important systemic component of the adaptive immune system that aid in the destruction of invaders. Immune repertoire refers to all the unique T-cell receptor (TCR) and B-cell receptor (BCR) genetic rearrangements. Only lymphocytes that encounter an antigen with the right receptor to bind to it will be activated and proliferate during an immune response, forming a clone of cells with identical antigen receptors for attack. A greater diversity of T-cell repertoire means there is higher likelihood for a T-cell to bind to the foreign entity (e.g. cancer cells) and increase the specific T-cell clonal population to destroy the invader.
Sequencing of the TCR beta chain CDR3 regions from all plasma samples and analyzed TCR repertoire diversity using the Shannon diversity index. TCR diversity was higher post-treatment compared to the pre-treatment. Boxes depict the interquartile range with the line in the boxes showing the median, and the lines outside the boxes show the first or third quartiles of fraction as shown in Figure 19.
Figure 19 — Increase in clonal diversity in blood of all treated patients, with much bigger difference in INT230-6 drug treated vs saline groups
The INVINCIBLE 2 study demonstrated feasibility, safety, tolerability and immune activation of presurgical IT injections in breast cancer patients. Preliminary data show histologic evidence of up to 95% tumor necrosis in varying
biologic subtypes including lobular carcinoma. There was also an increase in immune activation. Both of these study findings support the hypothesis that IT INT230-6 prior to SOC neoadjuvant therapy could increase pCR rates in high risk patients.
Planned Clinical Programs
Phase 3 Metastatic Soft Tissue Sarcoma (INVINCIBLE-3 Study)
Given the positive data on survival seen in our metastatic study in sarcoma patients, we plan to conduct a single Phase 3 study in 2nd/3rd line treatment for locally advanced, recurrent, inoperable, or metastatic non-diffuse soft tissue sarcoma with overall survival as the primary endpoint. The current Phase 3 study design plans to enroll subjects who will be randomized 2 to 1 to either INT230-6 for 5 doses Q2 weeks with maintenance dosing every 9 weeks for 2 years or the SOC. The three drugs most used for soft tissue sarcoma will be the control SOC at the investigator’s choice depending on the type of sarcoma. Our Phase 3 study is designed to be 90% powered to detect a difference Hazard Value of 0.65 in overall survival between the INT230-6 treatment group and the control group with 333 patients enrolled (2:1 randomization to either INT230-6 treatment or control therapy). The study will have 3 interim data reviews. The first at 20% of events (deaths) for futility only, the second at 40% of events, and the third at 60% of events. The final analysis will be based on 80% of events (266 deaths). A protocol synopsis was developed and submitted to the FDA. On October 14, 2021, we met with the FDA to discuss the Phase 3 protocol and reached alignment on the Phase 3 study design, patient population and statistical approach.. In November 2023, we submitted our IND to the FDA with the proposed full protocol. In December 2023, we received the Study May Proceed Letter from the FDA for our phase 3 trial in less than 30 days from the IND submission.
Figure 20 shows the survival curves from five recent Phase 3 studies using now approved SOC drugs for sarcoma, and also shows the expected Phase 3 survival for 1) the blended control based on the likely mix of sarcoma types (green curve) and 2) the expected INT230-6 Phase 3 survival curve that was generated based on our clinical results in sarcoma (navy blue curve). The references showing the Phase 3 data for the SOC controls are; for trabectadin: Patel S, et. Cancer. 2019 Aug 1;125(15):2610-2620; for eribulin: Schöffski et. al. Lancet. 2016 Apr 16;387(10028):1629-37; and for pazopanib: van der Graaf et. al. Lancet. 2012 May 19;379(9829):1879-86.
It is notable that despite different regimens and sarcoma subtype distributions, the overall survival is consistent for the current SOC drugs. Our Phase 2 program enrolled sarcoma patients with mixed subtypes whose cancer progressed despite a median of 3 prior treatments. We plan to enroll a similar mix of sarcoma patients; in Phase 3, however, no patient will have progressed on more than 2 treatments. Thus, patients in our planned Phase 3 study should be healthier than those treated in our Phase 2 study. Over 25% of patients in our Phase 2 study were well underdosed, and based on the tumor necrosis levels seen in IT-02, the dose loading into the tumor was insufficient for high levels of necrosis in the prior studies.
In the planned Phase 3 study, underdosing of patients will be less likely to occur, given dosing of INT230-6 can be as high as 175mL from day 1. Patients in the planned Phase 3 program shall also receive long term maintenance treatment of INT230-6 every 12 weeks, which mostly did not occur for many patients in our Phase 2 program.
Figure 20 — Survival curves of standard of care drugs and INT230-6 based on Phase 2
The survival curves from five recent Phase 3 studies using now approved standard of care drugs for sarcoma. The figure also shows the expected Phase 3 survival for 1) the blended control based on the likely mix of sarcoma types (green curve) and 2) the expected INT230-6 Phase 3 survival curve that was generated based on our clinical results in sarcoma (navy blue curve), which was conducted in a less healthy sarcoma population.
Figure 21 — The expected Phase 3 study schema comparing INT230-6 to the approved 2nd or 3rd line standard of care drugs
The current Phase 3 design proposes an endpoint of overall survival in a subset of advanced soft tissue sarcoma patients (leiomyosarcoma, liposarcoma and undifferentiated pleomorphic sarcoma). These subtypes comprise over 80% of the sarcoma populations. We plan to enroll two subjects in the INT230-6 group per one subject of any of the three SOC used drugs which will depend on the patient’s type of sarcoma. INT230-6 will be dosed every 2 weeks for 5 doses with maintenance every 12 weeks. The SOC drugs will be dosed at their approved regimens in each country. We anticipate 9 countries in North America, Europe and the Pacific will participate in this study.
Phase 2/3 Pre-surgical (Neoadjuvant) Triple Negative Breast (INVINCIBLE-4 or IT-04)
In November 2020, we met with the FDA to discuss use of our drug prior to surgery for breast cancer patients at high risk of disease recurrence such as those with triple negative breast cancer for potential accelerated approval.
The FDA instituted its Accelerated Approval Program to allow for earlier approval of drugs that treat serious conditions, and that fill an unmet medical need based on a surrogate endpoint. A surrogate endpoint is a marker, such as a laboratory measurement, radiographic image, physical sign or other measure that is thought to predict clinical benefit but is not itself a measure of clinical benefit. The use of a surrogate endpoint can considerably shorten the time required prior to receiving FDA approval. The surrogate endpoint we discussed with the FDA was pathological complete response.
Preoperative or neoadjuvant systemic chemotherapy, once reserved for patients with locally advanced breast cancer in whom the goal was to render large breast cancers operable, has become increasingly common. There are several potential reasons to consider neoadjuvant treatment for early-stage breast cancer. Giving chemotherapy preoperatively permits breast conservation in some patients who would otherwise require mastectomy and may improve cosmesis, or the preservation or restoration of physical appearance, in existing candidates for breast conservation. Preoperative therapy also provides a real-time evaluation of tumor response to permit discontinuation of ineffective therapy. Finally, the neoadjuvant setting offers investigators the unique opportunity to examine modulation of tissue, imaging, and other biomarkers from the time of biopsy to the time of definitive breast surgery following preoperative systemic therapy.
Pathological complete response (“pCR”) is an accepted FDA criteria for triple negative breast cancer for accelerated approval. pCR is defined as the absence of residual invasive and in situ cancer on H&E evaluation of the complete resected breast specimen and all sampled regional lymph nodes following completion of neoadjuvant systemic therapy.
On July 26, 2021, the FDA approved pembrolizumab (brand name Keytruda) for high-risk, early-stage, triple-negative breast cancer in combination with chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery.
The efficacy of pembrolizumab in combination with neoadjuvant chemotherapy followed by surgery and continued adjuvant treatment with pembrolizumab as a single agent was investigated in KEYNOTE-522, a randomized, multicenter, double-blind, placebo-controlled trial conducted in patients with newly diagnosed previously untreated high-risk early-stage triple-negative breast cancer. Patients were enrolled regardless of tumor PD-L1 expression. Patients were randomized (2:1) to pembrolizumab in combination with chemotherapy or placebo in combination with chemotherapy. The main efficacy outcome measures were pathological complete response rate and event free survival. The pathological complete response rate was only 63% for patients who received pembrolizumab in combination with chemotherapy compared with 56% for patients who received chemotherapy alone. However, that 7% increase in the pCR for pembrolizumab plus chemotherapy compared to chemotherapy alone was sufficient to result in a meaningful event free survival rate at 3 years for the 1200 patient Keynote 522 population. This in turn resulted in pembrolizumab receiving full approval for neoadjuvant and adjuvant use in triple negative breast cancer. At the same time, in Keynote 522 eighty percent (80%) of patients had a grade 3 or higher adverse event and 0.6% of patients died from the treatment.
As shown in Figure 17 above from the INVINCIBLE 2 Study INT230-6 can cause >95% of a large tumor to become necrotic on a single dose without toxicity other than minor pain at the injection site. Combining one or two doses upfront of INT230-6 with the SOC neoadjuvant therapy (pembrolizumab with anthracycline, cyclophosphamide and taxane) could potentially increase the pCR rate significantly to allow for accelerated approval especially in the more challenging tumors greater than or equal to 2 cm. Further use of INT230-6 may allow for the elimination of the anthracycline or cyclophosphamide and could reduce the toxicity of current chemotherapy regimen while obtaining an increase in pCR. The data on percent tumor necrosis form the phase 2 INVINCIBLE-4 Study will indicate how much necrosis can be induced upfront.
Following receipt of the final data from the phase 2 INVINCIBLE-4 Study, we plan to request another meeting with the FDA to review a Phase 3 randomized trial in neoadjuvant breast cancer with pCR improvement as the endpoint. The design of the Phase 2/3 study for presurgical use would be to add INT230-6 injection(s) in front of the SOC, which is chemotherapy with pembrolizumab, in TNBC and/or HER2+ versus SOC. The first endpoint would be for accelerated approval using Pathological Complete Response (pCR). Data for approval could be obtained 4 months post enrollment of the last patient. For full drug approval we would continue to enroll to show that the addition of INT230-6 to the SOC could lead to event free survival (EFS). The full approval endpoint would likely be at 3 years post-enrollment of the full EFS study. The Phase 2 design of the program is shown in Figure 22 below.
Figure 22 — The expected Phase 2 and 3 study schema comparing INT230-6 to the approved 2nd or 3rd line standard of care drugs
Phase 2 Metastatic Triple Negative Breast Cancer (contingent on additional capital raises).
The FDA designation of INT230-6 for Fast Track was made in 2018 as response to our proposed development program evaluating INT230-6 for the treatment of patients with relapsed or metastatic triple negative breast cancer.
Metastatic TNBC patients have a poor prognosis, with a median overall survival of 13.3 months with treatment first line. Recently approved treatments including Lynparza (PARP inhibitor) and Tecentriq (PD-L1 inhibitor). Those treatments target a specific subset of patients, with BRCA 1 or 2 and PD-L1 positive markers, respectively. Our target population would be more inclusive.
Continuing chemotherapy treatment until disease progression is currently the SOC for patients with metastatic TNBC, with no preferred chemotherapy regimens established at this time. Gilead presented data at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting (Abstract #1080) for second line use of sacituzumab (Trodelvy). Sacituzumab extended median overall survival to 10.9 months versus 4.9 months with chemotherapy (HR: 0.51; 95% CI: 0.28-0.91).
With a small sample size in study IT-01 INT230-6 either as monotherapy or with pembrolizumab has shown in refractory metastatic breast cancer (all types) a median overall survival of 12 months (n=9), and in subset of just m TNBC subjects, a median overall survival of approximately 12.5 months.
INT230-6 Phase 2/3 study design would consist of metastatic TNBC patients whose cancer has progressed following 1 to 2 lines of prior therapy. The Phase 2 study would be approximately 60 patients with INT230-6 arm and a control arm cohort design of patients using investigators choice of therapy. The endpoints would be median overall survival. Patients would receive 5 doses of INT230-6 every two weeks delivered IT with a maintenance dosing. The protocol will be designed to allow us to determine, within 12 months following completion of enrollment, whether INT230-6 has the potential to offer clinical benefit. A combination of INT230-6 with a checkpoint antibody (e.g. pembrolizumab or ipilimumab) within the randomized Phase 2 may be considered. From the results of the ongoing Phase 2, the company would make a strategic decision to use either monotherapy or combination with a checkpoint and size the final study accordingly. Phase 3 would be randomized 2 to 1 against investigators choice of treatment. Additional subjects could be added to the Phase 2 portion to complete the Phase 3 program. A clinical Phase 2/3 program in metastatic breast will be when sufficient capital is available post-initiation of the sarcoma Phase 3 study and the Phase 2/3 program in neoadjuvant breast cancer.
Government Regulation
The FDA and other regulatory authorities at federal, state and local levels, as well as in foreign countries, extensively regulate, among other things, the research, development, testing, manufacture, quality control, import, export, safety, effectiveness, labeling, packaging, storage, distribution, record keeping, approval, advertising, promotion, marketing, post-approval monitoring and post-approval reporting of drugs such as those we are developing. We, along with our vendors, collaboration partners, CROs and contract manufacturers, will be required to navigate the various preclinical, clinical,
manufacturing and commercial approval requirements of the governing regulatory agencies of the countries in which we wish to conduct studies or seek approval of our product candidate. The process of obtaining regulatory approvals of drugs and ensuring subsequent compliance with appropriate federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources.
In the United States, where we are initially focusing our product development, the FDA regulates drugs under the federal Food, Drug, and Cosmetic Act (“FDCA”) and its implementing regulations. Drug products are also subject to other federal, state and local statutes and regulations. Our product candidate is early-stage and has not been approved by the FDA for marketing in the United States.
The process required by the FDA before our product candidate is approved for therapeutic indications and may be marketed in the United States generally involves the following:
•completion of extensive preclinical studies in accordance with applicable regulations, including studies conducted in accordance with Good Laboratory Practice, or GLP, requirements;
•submission to the FDA of an IND, which must become effective before clinical trials may begin and must be updated annually or when significant changes are made;
•approval by an Institutional Review Board, or IRB, or independent ethics committee at each clinical trial site before each trial may be initiated;
•performance of adequate and well-controlled clinical trials in accordance with Good Clinical Practice, or GCP requirements and other clinical trial-related regulations to establish the safety and efficacy of the proposed drug product candidate for its intended purpose;
•preparation and submission to the FDA of a NDA after completion of all pivotal trials;
•a determination by the FDA of its receipt of an NDA, to file the application for review;
•satisfactory completion of one or more FDA pre-approval inspections of the manufacturing facility or facilities where the product will be produced to assess compliance with cGMP requirements, to assure that the facilities, methods and controls are adequate to assure the drug product’s identity, strength, quality and purity;
•potential FDA audit of the clinical trial sites that generated the data in support of the NDA;
•payment of user fees for FDA review of the NDA; and
•FDA review and approval of the NDA, including consideration of the views of any FDA advisory committee, prior to any commercial marketing or sale of the drug product in the United States.
Preclinical and clinical trials for drug products
Before testing any drug in humans, the product candidate must undergo rigorous preclinical testing. Preclinical studies include laboratory evaluations of chemistry, formulation and stability, as well as in vitro and animal studies to assess safety and in some cases to establish the rationale for therapeutic use. The conduct of preclinical studies is subject to federal and state regulations and requirements, including GLP requirements for safety and toxicology studies. The results of the preclinical studies, together with manufacturing information and analytical data must be submitted to the FDA as part of an IND. An IND is a request for authorization from the FDA to administer an investigational product to humans, and must become effective before clinical trials may begin. The central focus of an IND submission is on the general investigational plan and the protocol(s) for clinical studies. The IND also includes results of animal and in vitro studies assessing the toxicology, pharmacokinetics, pharmacology, and pharmacodynamic characteristics of the product; chemistry, manufacturing, and controls information; and any available human data or literature to support the use of the investigational product. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises concerns or questions about the conduct of the clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks, and imposes a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. Some long-term preclinical testing may continue after the IND is submitted. Accordingly, submission of an IND may or may not result in FDA authorization to begin a trial. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product development of a product candidate, and the FDA must grant permission, either explicitly or implicitly by not objecting, before each clinical trial can begin.
The clinical-stage of development involves the administration of the product candidate to healthy volunteers or patients under the supervision of qualified investigators, generally physicians not employed by or under the trial sponsor’s control, in accordance with GCP requirements, which include the requirements that all research subjects provide their
informed consent for their participation in any clinical trial. For cancer patients, the Phase 1 usually involves patients whose cancer has progressed following all approved therapies for that particular cancer.
Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria and the parameters and criteria to be used in monitoring safety and evaluating effectiveness. Each protocol, and any subsequent amendments to the protocol, must be submitted to the FDA as part of the IND. Furthermore, each clinical trial must be reviewed and approved by an IRB for each institution at which the clinical trial will be conducted to ensure that the risks to individuals participating in the clinical trials are minimized and are reasonable related to the anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical trial subject or his or her legal representative, and must monitor the clinical trial until completed. The FDA, the IRB, or the sponsor may suspend or discontinue a clinical trial at any time on various grounds, including a finding that the subjects are being exposed to an unacceptable health risk. Some studies also include oversight by an independent group of qualified experts organized by the clinical study sponsor, known as a data safety monitoring board, which provides authorization for whether or not a study may move forward at designated check points based on access to certain data from the study and may halt the clinical trial if it determines that there is an unacceptable safety risk for subjects or other grounds, such as no demonstration of efficacy. There also are requirements governing the reporting of ongoing clinical trials and completed clinical trials to public registries. Information about applicable clinical trials, including clinical trials results, must be submitted within specific timeframes for publication on the www.clinicaltrials.gov website. Disclosure of the results of such trials can be delayed in some cases for up to two years after the date of completion of the trial. Failure to timely register a covered clinical trial or to submit trial results as provided for in the law can give rise to civil monetary penalties and also prevent the non-compliant party from receiving future grant funds from the federal government. Both the NIH and the FDA have signaled willingness to enforce requirements under the NIH’s Final Rule on ClinicalTrials.gov registration and reporting requirements. Starting in 2021, the FDA has issued Notices of Non-Compliance to several companies in connection with ClinicalTrials.gov.
We have conducted our trials in Canada under a Clinical Trial Agreement with Health Canada, the regulatory authority in Canada. While we plan to conduct any international clinical trials under appropriate country filings in the future, a sponsor who wishes to conduct a clinical trial outside of the United States may, but need not, obtain FDA authorization to conduct the clinical trial under an IND. The FDA will accept a well-designed and well-conducted foreign clinical study not conducted under an IND if the study was conducted in accordance with GCP requirements, and the FDA is able to validate the data through an onsite inspection if deemed necessary.
Clinical trials to evaluate therapeutic indications to support NDAs for marketing approval are typically conducted in three sequential Phases, which may overlap.
•Phase 1 — Phase 1 clinical trials involve initial introduction of the investigational product into healthy human volunteers or patients with the target disease or condition. These studies are typically designed to test the safety, dosage tolerance, absorption, metabolism and distribution of the investigational product in humans, evaluate the side effects associated with increasing doses, and, if possible, to gain early evidence of effectiveness. As noted above for new cancer treatments such as ours, the Phase 1 involves patients whose cancer has progressed following all approved therapies for that particular cancer not healthy volunteers.
•Phase 2 — Phase 2 clinical trials typically involve administration of the investigational product to a limited patient population with a specified disease or condition to evaluate the preliminary efficacy, optimal dosages and dosing schedule and to identify possible adverse side effects and safety risks. Multiple Phase 2 clinical trials may be conducted to obtain information prior to beginning larger and more expensive Phase 3 clinical trials.
•Phase 3 — Phase 3 clinical trials typically involve administration of the investigational product to an expanded patient population to further evaluate dosage, to provide statistically significant evidence of clinical efficacy and to further test for safety, generally at multiple geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk/benefit ratio of the investigational product and to provide an adequate basis for product approval. Generally, one or two adequate and well-controlled Phase 3 clinical trials are required by the FDA for approval of an NDA.
Post-approval trials, sometimes referred to as Phase 4 clinical trials, may be conducted after initial marketing approval. These trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication and are commonly intended to generate additional safety data regarding use of the product in a clinical setting. In certain instances, the FDA may mandate the performance of Phase 4 clinical trials as a condition of approval of an NDA.
Progress reports detailing the results of the clinical trials, among other information, must be submitted at least annually to the FDA and written IND safety reports must be submitted to the FDA and the investigators fifteen days after
the trial sponsor determines the information qualifies for reporting for serious and unexpected suspected adverse events, findings from other studies or animal or in vitro testing that suggest a significant risk for human participants exposed to the investigational product and any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. The sponsor must also notify the FDA of any unexpected fatal or life-threatening suspected adverse reaction as soon as possible but in no case later than seven calendar days after the sponsor’s initial receipt of the information.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the drug characteristics of the product candidate and finalize a process for manufacturing the drug product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and manufacturers must develop, among other things, methods for testing the identity, strength, quality and purity of the final drug product. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life and to identify appropriate storage conditions for the product candidate.
NDA Submission and Review by the FDA
We intend to seek data exclusivity or market exclusivity for INT230-6. Assuming successful completion of the required clinical testing, the results of the preclinical studies and clinical trials, together with detailed information relating to the product’s chemistry, manufacture, controls and proposed labeling, among other things, are submitted to the FDA as part of an NDA requesting approval to market the product for one or more indications. An NDA is a request for approval to market a new drug for one or more specified indications. The NDA must include all relevant data available from pertinent pre-clinical and clinical studies, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls, and proposed labeling, among other things. Data may come from company-sponsored clinical trials intended to test the safety and efficacy of a product’s use or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and efficacy of the investigational product to the satisfaction of the FDA. FDA approval of an NDA must be obtained before a chemical drug may be marketed in the United States.
In addition, under the Pediatric Research Equity Act, or PREA, an NDA or supplement to an NDA must contain data to assess the safety and effectiveness of the drug product candidate for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The Food and Drug Administration Safety and Innovation Act requires that a sponsor who is planning to submit a marketing application for a drug product that includes a new clinically active component, new indication, new dosage form, new dosing regimen or new route of administration submit an initial Pediatric Study Plan (PSP) within sixty days after an end-of-Phase 2 meeting or as may be agreed between the sponsor and the FDA. The FDA may grant deferrals for submission of pediatric data or full or partial waivers. Unless otherwise required by regulation, PREA does not apply to any drug product for an indication for which orphan designation has been granted.
The FDA reviews all submitted NDAs before it accepts them for filing, and may request additional information rather than accepting the NDA for filing. The FDA must make a decision on accepting an NDA for filing within 60 days of receipt, and such decision could include a refusal to file by the FDA. Once the submission is accepted for filing, the FDA begins an in-depth substantive review of the NDA. The FDA reviews an NDA to determine, among other things, whether the product is safe and effective and whether the facility in which it is manufactured, processed, packaged or held meets standards designed to assure the product’s continued identity, strength, quality and purity. Under the goals and polices agreed to by the FDA under the Prescription Drug User Fee Act, or PDUFA, the FDA targets ten months, from the filing date, in which to complete its initial review of an original NDA and respond to the applicant, and six months from the filing date of an original NDA filed for priority review. The FDA does not always meet its PDUFA goal dates for standard or priority NDAs, and the review process is often extended by FDA requests for additional information or clarification.
Further, under PDUFA, as amended, each NDA must be accompanied by a substantial user fee, and the sponsor of an approved NDA is also subject to an annual program fee for each approved drug product. The FDA adjusts the PDUFA user fees on an annual basis. Fee waivers or reductions may be available in certain circumstances, including a waiver of the application fee for the first application filed by a small business. Additionally, no user fees are assessed on NDAs for products designated as orphan drugs, unless the product also includes a non-orphan indication.
The FDA may refer an application for a new drug product to an advisory committee. An advisory committee is a panel of independent experts, including clinicians and other scientific experts, which reviews, evaluates and provides a
recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA may inspect one or more clinical trial sites to assure compliance with GCP and other requirements and the integrity of the clinical data submitted to the FDA.
The FDA also may require submission of a Risk Evaluation and Mitigation Strategy, or REMS, as a condition for approving the NDA to ensure that the benefits of the product outweigh its risks. The REMS could include medication guides, physician communication plans, assessment plans, and/or elements to assure safe use, such as restricted distribution methods, patient registries, or other risk-minimization tools.
After evaluating the NDA and all related information, including the advisory committee recommendation, if any, and inspection reports regarding the manufacturing facilities and clinical trial sites, the FDA may issue an approval letter or a Complete Response Letter. A Complete Response Letter indicates that the review cycle of the application is complete and the application is not ready for approval. A Complete Response Letter will usually describe all of the deficiencies that the FDA has identified in the NDA, except that where the FDA determines that the data supporting the application are inadequate to support approval, the FDA may issue the Complete Response Letter without first conducting required inspections, testing submitted product lots, and/or reviewing proposed labeling. In issuing the Complete Response Letter, the FDA may recommend actions that the applicant might take to place the NDA in condition for approval, including requests for additional information or clarification. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval. If and when those conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications.
Even if the FDA approves a product, depending on the specific risk(s) to be addressed, the FDA may limit the approved indications for use of the product, require that contraindications, warnings or precautions be included in the product labeling, require that post-approval studies, including Phase 4 clinical trials, be conducted to further assess a product’s safety after approval, require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution and use restrictions or other risk management mechanisms under a REMS, which can materially affect the potential market and profitability of the product. The FDA may prevent or limit further marketing of a product based on the results of post-marketing studies or surveillance programs. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes, and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Expedited Development and Review Programs for Drugs
The FDA maintains several programs intended to facilitate and expedite development and review of new drugs to address unmet medical needs in the treatment of serious or life-threatening diseases or conditions. These programs include Fast Track Designation, Breakthrough Therapy designation, priority review and Accelerated Approval.
A new drug product is eligible for Fast Track Designation if it is intended to treat a serious or life-threatening disease or condition and demonstrates the potential to address unmet medical needs for such disease or condition. Fast Track Designation applies to the combination of the product and the specific indication for which it is being studied. Fast Track Designation provides increased opportunities for sponsor interactions with the FDA during preclinical and clinical development, in addition to the potential for rolling review once a marketing application is filed, meaning that the FDA may consider for review sections of the NDA on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the NDA, the FDA agrees to accept sections of the NDA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the NDA.
In addition, a new drug product may be eligible for Breakthrough Therapy designation if it is intended to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the drug, alone or in combination with one or more other drugs or biologics, may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Breakthrough Therapy designation provides all the features of Fast Track Designation in addition to intensive guidance on an efficient development program beginning as early as Phase 1, and FDA organizational commitment to expedited development, including involvement of senior managers and experienced review staff in a cross-disciplinary review, where appropriate.
Any product submitted to the FDA for approval, including a product with Fast Track or Breakthrough Therapy designation, may also be eligible for additional FDA programs intended to expedite the review and approval process, including priority review and Accelerated Approval. A product is eligible for priority review if it is intended to treat a serious or life-threatening disease or condition, and if approved, would provide a significant improvement in safety or effectiveness. For original NDAs, priority review designation means the FDA’s goal is to take action on the marketing application within six months of the 60-day filing date (compared with ten months under standard review).
A product intended to treat serious or life-threatening diseases or conditions may receive Accelerated Approval upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than on irreversible morbidity or mortality which is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments.
Accelerated Approval is usually contingent on a sponsor’s agreement to conduct additional post-approval studies to verify and describe the product’s clinical benefit. The FDA may withdraw approval of a drug approved under Accelerated Approval if, for example, the sponsor fails to conduct the confirmatory trials in a timely manner or the confirmatory trial fails to verify the predicted clinical benefit of the product. In addition, unless otherwise informed by the FDA, the FDA currently requires, as a condition for Accelerated Approval, that all advertising and promotional materials that are intended for dissemination or publication within 120 days following marketing approval be submitted to the agency for review during the pre-approval review period, and that after 120 days following marketing approval, all advertising and promotional materials must be submitted at least 30 days prior to the intended time of initial dissemination or publication.
Fast Track Designation, Breakthrough Therapy designation, priority review and Accelerated Approval do not change the scientific or medical standards for approval or the quality of evidence necessary to support approval but may expedite the development or review process.
U.S. Post-Approval Requirements for Drugs
Drugs manufactured or distributed pursuant to the FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, reporting of adverse experiences with the product, complying with promotion and advertising requirements, which include restrictions on promoting products for unapproved uses or patient populations (known as “off-label use”) and limitations on industry-sponsored scientific and educational activities. Although physicians may prescribe approved products for off-label uses, manufacturers may not market or promote such uses. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, including not only by our employees but also by agents of us or those speaking on our behalf, and a company that is found to have improperly promoted off-label uses may be subject to significant liability. Failure to comply with these requirements can result in, among other things, adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties, including liabilities under the False Claims Act where products carry reimbursement under federal health care programs. Promotional materials for approved drugs must be submitted to the FDA in conjunction with their first use or first publication. Further, if there are any modifications to the product, including changes in indications, labeling or manufacturing processes or facilities, the applicant may be required to submit and obtain FDA approval of a new NDA or NDA supplement, which may require the development of additional data or preclinical studies and clinical trials.
The FDA may impose a number of post-approval requirements as a condition of approval of an NDA. For example, the FDA may require post-market testing, including Phase 4 clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization.
In addition, drug manufacturers and their subcontractors involved in the manufacture and distribution of approved products are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMP, which impose certain procedural and documentation requirements upon us and our contract manufacturers. Changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting requirements upon us and any third-party manufacturers that we may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance. Failure to comply with statutory and regulatory requirements can subject a manufacturer to possible legal or regulatory action, such as warning letters, suspension of manufacturing, product seizures, injunctions, civil penalties or criminal prosecution. There is also a continuing, annual program fee for any marketed product.
The FDA may withdraw approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information, requirements for post-market studies or clinical trials to assess new safety risks, or imposition of distribution or other restrictions under a REMS. Other potential consequences include, among other things:
•restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls;
•safety alerts, Dear Healthcare Provider letters, press releases or other communications containing warnings or other safety information about the product;
•mandated modification of promotional materials and labeling and issuance of corrective information;
•fines, warning letters, or untitled letters;
•holds on clinical trials;
•refusal of the FDA to approve applications or supplements to approved applications, or suspension or revocation of product approvals;
•product seizure or detention, or refusal to permit the import or export of products;
•injunctions or the imposition of civil or criminal penalties; and
•consent decrees, corporate integrity agreements, debarment or exclusion from federal healthcare programs.
Orphan Designation and Exclusivity
Under the Orphan Drug Act, the FDA may grant orphan drug designation, or ODD, to a drug or biologic intended to treat a rare disease or condition, defined as a disease or condition with either a patient population of fewer than 200,000 individuals in the United States, or a patient population greater of than 200,000 individuals in the United States when there is no reasonable expectation that the cost of developing and making available the drug or biologic in the United States will be recovered from sales in the United States of that drug or biologic. ODD must be requested before submitting an NDA. After the FDA grants ODD, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA.
If a product that has received ODD and subsequently receives the first FDA approval for a particular clinically active component for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a full NDA, to market the same biologic for the same indication for seven years from the approval of the NDA, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity or if the FDA finds that the holder of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or condition for which the drug was designated. Orphan drug exclusivity does not prevent the FDA from approving a different drug or biologic for the same disease or condition, or the same drug or biologic for a different disease or condition. Among the other benefits of ODD are tax credits for certain research and a waiver of the NDA application user fee.
A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received ODD. In addition, orphan drug exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
A drug product can also obtain pediatric market exclusivity in the United States. Pediatric exclusivity, if granted, adds six months to existing exclusivity periods and patent terms. This six-month exclusivity, which runs from the end of other exclusivity protection or patent term, may be granted based on the voluntary completion of a pediatric study in accordance with an FDA-issued “Written Request” for such a study. The data from such study do not need to show the product to be effective in the pediatric population studied; rather, if the clinical trial is deemed to fairly respond to the FDA’s request, the additional protection is granted. Although this is not a patent term extension, it effectively extends the regulatory period during which the FDA cannot approve another application. We filed for orphan drug status with the FDA in December 2021, responded to clarifications from the FDA in March 2022, and received orphan drug designation for all three components of INT230-6 SHAO, cisplatin and vinblastine, for soft tissue sarcoma in June 2022. This designation
grants the company seven years of marketing exclusivity following approval in the soft tissue sarcoma for any of our products containing any one of these three ingredients.
The Hatch-Waxman Act and Marketing Exclusivity
Under the Drug Price Competition and Patent Term Restoration Act of 1984, otherwise known as the Hatch-Waxman Amendments to the FDCA, Congress authorized the FDA to approve generic drugs that are the same as drugs previously approved by the FDA under the NDA provisions of the statute and also enacted Section 505(b)(2) of the FDCA. To obtain approval of a generic drug, an applicant must submit an abbreviated new drug application, or ANDA, to the agency. In support of such applications, a generic manufacturer may rely on the preclinical and clinical testing conducted for a drug product previously approved under an NDA, known as the reference listed drug (RLD). Specifically, in order for an ANDA to be approved, the FDA must find that the generic version is identical to the RLD with respect to the active ingredients, the route of administration, the dosage form, and the strength of the drug. In contrast, Section 505(b)(2) permits the filing of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. A Section 505(b)(2) applicant may eliminate the need to conduct certain preclinical or clinical studies, if it can establish that reliance on studies conducted for a previously approved product is scientifically appropriate. Unlike the ANDA pathway used by developers of bioequivalent versions of innovator drugs, which does not allow applicants to submit new clinical data other than bioavailability or bioequivalence data, the 505(b)(2) regulatory pathway does not preclude the possibility that a follow-on applicant would need to conduct additional clinical trials or nonclinical studies; for example, they may be seeking approval to market a previously approved drug for new indications or for a new patient population that would require new clinical data to demonstrate safety or effectiveness. The FDA may then approve the new product for all or some of the label indications for which the RLD has been approved, or for any new indication sought by the Section 505(b)(2) applicant, as applicable.
In seeking approval of an NDA or a supplement thereto, the NDA sponsor is required to list with the FDA each patent with claims that cover the sponsor’s product or an approved method of using the product. Upon approval of an NDA, each of the patents listed in the application for the drug is published in the FDA publication Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. When an ANDA applicant submits its application to the FDA, the applicant is required to certify to the FDA concerning any patents listed in the Orange Book for the RLD, except for patents covering methods of use for which the follow-on applicant is not seeking approval. To the extent a Section 505(b)(2) applicant is relying on studies conducted for an already approved product, such an applicant is also required to certify to the FDA concerning any patents listed for the approved product in the Orange Book to the same extent that an ANDA applicant would.
Specifically, any applicant who subsequently files an ANDA or 505(b)(2) NDA that references the drug listed in the Orange Book must certify to the FDA that with respect to each published patent, (i) the required patent information has not been filed by the original applicant of the RLD; (ii) the listed patent already has expired; (iii) the listed patent has not expired, but will expire on a specified date and approval is sought after patent expiration; or (iv) the listed patent is invalid, unenforceable or will not be infringed by the manufacture, use or sale of the new product. These are known as Paragraph I, II, III, and IV certifications, respectively.
If a Paragraph I or II certification is filed, the FDA may make approval of the application effective immediately upon completion of its review. If a Paragraph III certification is filed, the approval may be made effective on the patent expiration date specified in the application, although a tentative approval may be issued before that time. If an application contains a Paragraph IV certification, a series of events will be triggered, the outcome of which will determine the effective date of approval of the ANDA or 505(b)(2) application.
A certification that the new product will not infringe the RLD’s listed patents or that such patents are invalid is called a Paragraph IV certification. If the follow-on applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders for the RLD once the applicant’s NDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a legal challenge to the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of their receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA or 505(b)(2) NDA until the earlier of 30 months after the receipt of the Paragraph IV notice, expiration of the patent or a decision in the infringement case that is favorable to the ANDA or 505(b)(2) applicant. Alternatively, if the listed patent holder does not file a patent infringement lawsuit within the required 45-day period, the follow-on applicant’s ANDA or 505(b)(2) NDA will not be subject to the 30-month stay.
In addition, under the Hatch-Waxman Amendments, the FDA may not approve an ANDA or 505(b)(2) NDA until any applicable period of non-patent exclusivity for the referenced RLD has expired. These market exclusivity provisions under the FDCA also can delay the submission or the approval of certain applications. The FDCA provides a five-year
period of non-patent marketing exclusivity within the United States to the first applicant to gain approval of an NDA for a drug containing a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an ANDA or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement.
The FDCA also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the conditions of use associated with the new clinical investigations and does not prohibit the FDA from approving follow-on applications for drugs containing the original active agent. Five-year and three-year exclusivity also will not delay the submission or approval of a traditional NDA filed under Section 505(b)(1) of the FDCA. However, an applicant submitting a traditional NDA would be required to either conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Patent Term Extension
After NDA approval, owners of relevant drug patents may apply for up to a five-year patent term extension. The allowable patent term extension is calculated as half of the drug’s testing phase — the time between when the IND becomes effective and NDA submission — and all of the review phase — the time between NDA submission and approval, up to a maximum of five years. The time can be shortened if the FDA determines that the applicant did not pursue approval with due diligence. The total patent term after the extension may not exceed 14 years. For patents that might expire during the application phase, the patent owner may request an interim patent extension. An interim patent extension increases the patent term by one year and may be renewed up to four times. For each interim patent extension granted, the post-approval patent extension is reduced by one year. The director of the Patent and Trademark Office (PTO) must determine that approval of the drug covered by the patent for which a patent extension is being sought is likely. Interim patent extensions are not available for a drug for which an NDA has not been submitted.
Other Regulatory Matters
Manufacturing, sales, promotion and other activities of drug products following approval, where applicable, or commercialization are also subject to regulation by numerous regulatory authorities in the United States in addition to the FDA, which may include the Centers for Medicare & Medicaid Services, or CMS, other divisions of the Department of Health and Human Services, or HHS, the Department of Justice, the Drug Enforcement Administration, the Consumer Product Safety Commission, the Federal Trade Commission, the Occupational Safety & Health Administration, the Environmental Protection Agency and state and local governments and governmental agencies.
Other Healthcare Laws
Healthcare providers, physicians, and third-party payors will play a primary role in the recommendation and prescription of any products for which we obtain marketing approval. Our business operations and any current or future arrangements with third-party payors, healthcare providers and physicians may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we develop, market, sell and distribute any drugs for which we obtain marketing approval. In the United States, these laws include, without limitation, state and federal anti-kickback, false claims, physician transparency, and patient data privacy and security laws and regulations, including but not limited to those described below.
•The federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, paying, receiving or providing any remuneration (including any kickback, bribe, or certain rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid; a person or entity need not have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it in order to have committed a violation. Violations are subject to civil and criminal fines and penalties for each violation, plus up to three times the remuneration involved, imprisonment, and exclusion from government healthcare programs;
•The federal civil and criminal false claims laws, including the civil False Claims Act, or FCA, which prohibit individuals or entities from, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment or approval that are false, fictitious or fraudulent; knowingly making, using, or causing to be made or used, a false statement or record material to a false or fraudulent claim or obligation to pay or transmit money or property to the federal government; or knowingly concealing or knowingly and improperly avoiding or decreasing an obligation to pay money to the federal government. Manufacturers can be held liable under the FCA even when they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent claims. In addition, the government may assert that a claim that includes items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act. The FCA also permits a private individual acting as a “whistleblower” to bring actions on behalf of the federal government alleging violations of the FCA and to share in any monetary recovery. When an entity is determined to have violated the federal civil False Claims Act, the government may impose civil fines and penalties for each false claim, plus treble damages, and exclude the entity from participation in Medicare, Medicaid and other federal healthcare programs;
•The federal civil monetary penalties laws, which impose civil fines for, among other things, the offering or transfer or remuneration to a Medicare or state healthcare program beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner, or supplier of services reimbursable by Medicare or a state health care program, unless an exception applies;
•The Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for knowingly and willfully executing a scheme, or attempting to execute a scheme, to defraud any healthcare benefit program, including private payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, or falsifying, concealing or covering up a material fact or making any materially false statements in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the federal Anti-Kickback Statute, a person or entity need not have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
•HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, imposes, among other things, specified requirements on covered entities and their business associates relating to the privacy and security of individually identifiable health information including mandatory contractual terms and required implementation of technical safeguards of such information. HITECH also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates in some cases, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions;
•The Physician Payments Sunshine Act, enacted as part of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively, the ACA, imposed new annual reporting requirements for certain manufacturers of drugs, devices, biologics, and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, for certain payments and “transfers of value” provided to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. In addition, many states also require reporting of payments or other transfers of value, many of which differ from each other in significant ways, are often not pre-empted, and may have a more prohibitive effect than the Sunshine Act, thus further complicating compliance efforts. Effective January 1, 2022, these reporting obligations will extend to include transfers of value made in the previous year to certain non-physician providers such as physician assistants and nurse practitioners;
•Federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; and
•Analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third party-payors, including private insurers, and may be broader in scope than their federal equivalents; state and foreign laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; state and foreign laws
that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers, and restrict marketing practices or require disclosure of marketing expenditures and pricing information; state and foreign laws that govern the privacy and security of health information in some circumstances. These data privacy and security laws may differ from each other in significant ways and often are not pre-empted by HIPAA, which may complicate compliance efforts.
The distribution of drug products is subject to additional requirements and regulations, including extensive record-keeping, licensing, storage and security requirements intended to prevent the unauthorized sale of pharmaceutical products.
The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Federal and state enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. It is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other related governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, disgorgement, exclusion from government funded healthcare programs, such as Medicare and Medicaid, reputational harm, additional oversight and reporting obligations if we become subject to a corporate integrity agreement or similar settlement to resolve allegations of non-compliance with these laws and the curtailment or restructuring of our operations. If any of the physicians or other healthcare providers or entities with whom we expect to do business are found to be not in compliance with applicable laws, they may be subject to similar actions, penalties and sanctions. Ensuring business arrangements comply with applicable healthcare laws, as well as responding to possible investigations by government authorities, can be time- and resource-consuming and can divert a company’s attention from its business.
Coverage and Reimbursement
In the United States and markets in other countries, patients who are prescribed treatments for their conditions and providers performing the prescribed services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Thus, even if a product candidate is approved, sales of the product will depend, in part, on the extent to which third-party payors, including government health programs in the United States such as Medicare and Medicaid, commercial health insurers and managed care organizations, provide coverage, and establish adequate reimbursement levels for, the product. In the United States, no uniform policy of coverage and reimbursement for drug products exists among third-party payors. Therefore, coverage and reimbursement for drug products can differ significantly from payor to payor. The process for determining whether a third-party payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved. Third-party payors are increasingly challenging the prices charged, examining the medical necessity, and reviewing the cost-effectiveness of medical products and services and imposing controls to manage costs. Third-party payors may limit coverage to specific products on an approved list, also known as a formulary, which might not include all of the approved products for a particular indication.
In order to secure coverage and reimbursement for any product that might be approved for sale, a company may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costs required to obtain FDA or other comparable regulatory approvals. Additionally, companies may also need to provide discounts to purchasers, private health plans or government healthcare programs. Nonetheless, product candidates may not be considered medically necessary or cost effective. A decision by a third-party payor not to cover a product could reduce physician utilization once the product is approved and have a material adverse effect on sales, our operations and financial condition. Additionally, a third-party payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage and reimbursement for the product, and the level of coverage and reimbursement can differ significantly from payor to payor.
The containment of healthcare costs has become a priority of federal, state and foreign governments, and the prices of products have been a focus in this effort. Governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit a company’s revenue generated from the sale of any approved products. Coverage policies and third-party payor reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which a company or its collaborators receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Healthcare Reform
In the United States and some foreign jurisdictions, there have been, and likely will continue to be, a number of legislative and regulatory changes and proposed changes regarding the healthcare system directed at broadening the availability of healthcare, improving the quality of healthcare, and containing or lowering the cost of healthcare. For example, in March 2010, the United States Congress enacted the ACA, which, among other things, includes changes to the coverage and payment for products under government health care programs. The ACA includes provisions of importance to our potential product candidate that:
•created an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic products, apportioned among these entities according to their market share in certain government healthcare programs;
•expanded eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to certain individuals with income at or below 133% of the federal poverty level, thereby potentially increasing a manufacturer’s Medicaid rebate liability;
•expanded manufacturers’ rebate liability under the Medicaid Drug Rebate Program by increasing the minimum rebate for both branded and generic drugs and revising the definition of “average manufacturer price,” or AMP, for calculating and reporting Medicaid drug rebates on outpatient prescription drug prices;
•addressed a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected;
•expanded the types of entities eligible for the 340B drug discount program;
•established the Medicare Part D coverage gap discount program by requiring manufacturers to provide point-of-sale-discounts off the negotiated price of applicable brand drugs to eligible beneficiaries during their coverage gap period as a condition for the manufacturers’ outpatient drugs to be covered under Medicare Part D; and
•created a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research.
Since its enactment, there have been numerous judicial, administrative, executive, and legislative challenges to certain aspects of the ACA. For example, the Tax Cuts and Jobs Act of 2017 (the Tax Act) was enacted, which includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” On December 14, 2018, a U.S. District Court Judge in the Northern District of Texas ruled that the individual mandate is a critical and inseverable feature of the ACA, and therefore, because it was repealed as part of the Tax Act, the remaining provisions of the ACA are invalid as well. Additionally, on December 18, 2019, the U.S. Court of Appeals for the 5th Circuit ruled that that the individual mandate was unconstitutional and remanded the case back to the District Court to determine whether the remaining provisions of the ACA are invalid as well. The U.S. Supreme Court held in a 7 – 2 opinion that the states and individuals that brought the lawsuit challenging the ACA’s individual mandate do not have standing to challenge the law. The Supreme Court did not reach the merits of the challenge, but the decision ends the case. It is also unclear how the Supreme Court ruling, other such litigation, and the healthcare reform measures of the Biden administration will impact the ACA or our business.
Other legislative changes have been proposed and adopted in the United States since the Affordable Care Act was enacted. In August 2011, the Budget Control Act of 2011, among other things, included aggregate reductions of Medicare payments to providers of 2% per fiscal year, which went into effect in April 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2031 unless additional Congressional action is taken. The Coronavirus Aid, Relief, and Economic Security Act, or CARES Act, which was signed into law on March 27, 2020, designed to provide financial support and resources to individuals and businesses affected by the COVID-19 pandemic, suspended these reductions from May 1, 2020 through December 31, 2020, and extended the sequester by one year, through 2030. In addition, in January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. Additionally, on March 11, 2021, President Biden signed the American Rescue Plan Act of 2021 into law, which eliminates the statutory Medicaid drug rebate cap, currently set at 100% of a drug’s average manufacturer price, for single-source and innovator multiple-source drugs, beginning January 1, 2024. These laws may result in additional reductions in Medicare, Medicaid and other healthcare funding.
Moreover, payment methodologies may be subject to changes in healthcare legislation and regulatory initiatives. For example, CMS may develop new payment and delivery models, such as bundled payment models. In addition, recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their commercial products, which has resulted in several Congressional inquiries and proposed and enacted state and federal legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for pharmaceutical products. For example, on July 24, 2020 and September 13, 2020, the Trump administration announced several executive orders related to prescription drug pricing and importation. As a result, the FDA also released a final rule in September 2020, effective November 30, 2020, providing guidance for states to build and submit importation plans for drugs from Canada. Further, in November 2020, the U.S. Department of Health and Human Services, or HHS, finalized a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The implementation of the rule has been postponed by Congress and HHS to January 1, 2032.
The CMS also issued an interim final rule that would have established a Most Favored Nation, or MFN, Model for Medicare Part B drug payments. This regulation would have substantially changed the reimbursement landscape as it based Medicare Part B payment for 50 selected drugs on prices in foreign countries instead of average sales prices (ASP) and established a fixed add-on payment in place of the current 6 percent (4.3 percent after sequestration) of ASP. The MFN drug payment amount was expected to be lower than the current ASP-based limit because U.S. drug prices are generally the highest in the world. However, CMS issued a final rule on December 29, 2021 that rescinded the MFN Model interim final rule.
In December 2020, CMS issued a final rule implementing significant manufacturer price reporting changes under the Medicaid Drug Rebate Program, including regulations that affect manufacturer-sponsored patient assistance programs subject to pharmacy benefit manager accumulator programs and Best Price reporting related to certain value-based purchasing arrangements. On May 21, 2021, an industry group sued CMS, claiming that the change to the Best Price rule exceeds CMS’s statutory authority and is contrary to the Medicaid Rebate statute, and on May 17, 2022, the U.S. District Court for the District of Columbia vacated the Best Price rule.
In August 2022, President Biden signed the Inflation Reduction Act of 2022 (IRA) into law. This statute marks the most significant action by Congress with respect to the pharmaceutical industry since adoption of the ACA in 2010. Among other things, the IRA requires manufacturers of certain drugs to engage in price negotiations with Medicare (beginning in 2026), with prices that can be negotiated subject to a cap; imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation (beginning October 1, 2022); and replaces the Medicare Part D coverage gap discount program with a new discounting program (beginning in 2025). The IRA permits the Secretary of the Department of Health and Human Services (HHS) to implement many of these provisions through guidance, as opposed to regulation, for the initial years. On March 15, 2023, and June 30, 2023, HHS issued guidance regarding implementation of the Medicare drug price negotiation program in initial price applicability year 2026. HHS stated it would provide additional information in the future related to implementation for initial price applicability years 2027 and beyond. Some provisions of the IRA may be subject to legal challenge. Several manufacturers and industry groups have challenged the drug price negotiation program for Medicare Parts B and D in federal court. These lawsuits are ongoing, and additional lawsuits may be filed in the future. It is unknown whether such litigation or other litigation, if brought, will be successful. For these and other reasons, it is currently unclear how the IRA will be effectuated, and while the impact of the IRA on the pharmaceutical industry cannot yet be fully determined, it is likely to be significant.
On May 23, 2023, CMS issued a proposed rule that would modify many Medicaid Drug Rebate Program requirements and implement a drug price verification survey. It is unclear whether the proposed rule will be finalized and whether the final rule will differ from the proposed rule. It is also unclear to what extent these regulations or any future legislation or regulations will have on our business, including our ability to generate revenue and achieve profitability.
On May 30, 2018, the Right to Try Act was signed into law. The law, among other things, provides a federal framework for certain patients to access certain investigational new drug products that have completed a Phase 1 clinical trial and that are undergoing investigation for FDA approval. Under certain circumstances, eligible patients can seek treatment without enrolling in clinical trials and without obtaining FDA permission under the FDA expanded access program. There is no obligation for a drug manufacturer to make its drug products available to eligible patients as a result of the Right to Try Act, but the manufacturer must develop an internal policy and respond to patient requests according to that policy.
Outside the United States, ensuring coverage and adequate payment for a product also involves challenges. Pricing of prescription pharmaceuticals is subject to government control in many countries. Pricing negotiations with government authorities can extend well beyond the receipt of regulatory approval for a product and may require a clinical trial that
compares the cost-effectiveness of a product to other available therapies. The conduct of such a clinical trial could be expensive and result in delays in commercialization.
In the European Union, pricing and reimbursement schemes vary widely from country to country. Some countries provide that products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to currently available therapies or so-called health technology assessments, in order to obtain reimbursement or pricing approval. For example, the European Union provides options for its member states to restrict the range of products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. European Union member states may approve a specific price for a product or they may instead adopt a system of direct or indirect controls on our profitability for placing the product on the market. Other member states allow companies to fix their own prices for products, but monitor and control prescription volumes and issue guidance to physicians to limit prescriptions. Recently, many countries in the European Union have increased the amount of discounts required on pharmaceuticals and these efforts could continue as countries attempt to manage healthcare expenditures, especially in light of the severe fiscal and debt crises experienced by many countries in the European Union. The downward pressure on healthcare costs in general, particularly prescription products, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various European Union member states, and parallel trade, i.e., arbitrage between low-priced and high-priced member states, can further reduce prices. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any products, if approved in those countries.
Compliance with Other Federal and State Laws or Requirements; Changing Legal Requirements
If any products that we may develop are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. Products must meet applicable child-resistant packaging requirements under the U.S. Poison Prevention Packaging Act. Manufacturing, labeling, packaging, distribution, sales, promotion and other activities also are potentially subject to federal and state consumer protection and unfair competition laws, among other requirements to we may be subject.
The distribution of pharmaceutical products is subject to additional requirements and regulations, including extensive record-keeping, licensing, storage and security requirements intended to prevent the unauthorized sale of pharmaceutical products.
The failure to comply with any of these laws or regulatory requirements subjects firms to possible legal or regulatory action. Depending on the circumstances, failure to meet applicable regulatory requirements can result in criminal prosecution, fines or other penalties, injunctions, exclusion from federal healthcare programs, requests for recall, seizure of products, total or partial suspension of production, denial or withdrawal of product approvals, relabeling or repackaging, or refusal to allow a firm to enter into supply contracts, including government contracts. Any claim or action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Prohibitions or restrictions on marketing, sales or withdrawal of future products marketed by us could materially affect our business in an adverse way.
Changes in regulations, statutes or the interpretation of existing regulations could impact our business in the future by requiring, for example: (i) changes to our manufacturing arrangements; (ii) additions or modifications to product labeling or packaging; (iii) the recall or discontinuation of our product candidates; or (iv) additional record-keeping requirements. If any such changes were to be imposed, they could adversely affect the operation of our business.
Other U.S. Environmental, Health and Safety Laws and Regulations
We may be subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. From time to time and in the future, our operations may involve the use of hazardous and flammable materials, including chemicals and drug materials, and may also produce hazardous waste products. Even if we contract with third parties for the disposal of these materials and waste products, we cannot completely eliminate the risk of contamination or injury resulting from these materials. In the event of contamination or injury resulting from the use or disposal of our hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with such laws and regulations.
We maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees, but this insurance may not provide adequate coverage against potential liabilities. However, we do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. Current or future environmental laws and regulations may impair our research, development or production efforts. In addition, failure to comply with these laws and regulations may result in substantial fines, penalties or other sanctions.
Government Regulation of Drugs Outside of the United States
To market any product outside of the United States, we would need to comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy and governing, among other things, clinical trials, marketing authorization or identification of an alternate regulatory pathway, manufacturing, commercial sales and distribution of our product candidates. For instance, in the European Economic Area, or the EEA (comprised of the 26 EU Member States plus Iceland, Liechtenstein and Norway, with the UK having left the EU in January of 2020), medicinal products must be authorized for marketing by using either the centralized authorization procedure or national authorization procedures.
•Centralized procedure — If pursuing marketing authorization of a product candidate for a therapeutic indication under the centralized procedure, following the opining of the EMA’s Committee for Medicinal Products for Human Use, or, CHMP, the European Commission issues a single marketing authorization valid across the EEA. The centralized procedure is compulsory for human medicines derived from biotechnology processes or advanced therapy medicinal products (such as gene therapy, somatic cell therapy and tissue engineered products), products that contain a new active substance indicated for the treatment of certain diseases, such as HIV/AIDS, cancer, neurodegenerative disorders, diabetes, autoimmune diseases and other immune dysfunctions, viral diseases, and officially designated orphan medicines. For medicines that do not fall within these categories, an applicant has the option of submitting an application for a centralized marketing authorization to the EMA, as long as the medicine concerned contains a new active substance not yet authorized in the EEA, is a significant therapeutic, scientific or technical innovation, or if its authorization would be in the interest of public health in the EEA. Under the centralized procedure the maximum timeframe for the evaluation of an MAA by the EMA is 210 days, excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the CHMP. Accelerated assessment might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a major public health interest, particularly from the point of view of therapeutic innovation. The timeframe for the evaluation of an MAA under the accelerated assessment procedure is 150 days, excluding clock stops.
•National authorization procedures — There are also two other possible routes to authorize products for therapeutic indications in several countries, which are available for products that fall outside the scope of the centralized procedure:
•Decentralized procedure — Using the decentralized procedure, an applicant may apply for simultaneous authorization in more than one EU country of medicinal products that have not yet been authorized in any EU country and that do not fall within the mandatory scope of the centralized procedure.
•Mutual recognition procedure — In the mutual recognition procedure, a medicine is first authorized in one EU Member State, in accordance with the national procedures of that country. Following this, additional marketing authorizations can be sought from other EU countries in a procedure whereby the countries concerned recognize the validity of the original, national marketing authorization.
In the EEA, new products for therapeutic indications that are authorized for marketing (i.e., reference products) qualify for eight years of data exclusivity and an additional two years of market exclusivity upon marketing authorization. The data exclusivity period prevents generic or biosimilar applicants from relying on the preclinical and clinical trial data contained in the dossier of the reference product when applying for a generic or biosimilar marketing authorization in the EU during a period of eight years from the date on which the reference product was first authorized in the EU. The market exclusivity period prevents a successful generic or biosimilar applicant from commercializing its product in the EU until ten years have elapsed from the initial authorization of the reference product in the EU. The ten-year market exclusivity period can be extended to a maximum of eleven years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies.
Similar to the United States, the various phases of non-clinical and clinical research in the European Union are subject to significant regulatory controls.
The Clinical Trials Directive 2001/20/EC, the Directive 2005/28/EC on GCP and the related national implementing provisions of the individual EU Member States govern the system for the approval of clinical trials in the European Union. Under this system, an applicant must obtain prior approval from the competent national authority of the EU Member States in which the clinical trial is to be conducted. Furthermore, the applicant may only start a clinical trial at a specific study site after the competent ethics committee has issued a favorable opinion. The clinical trial application must be accompanied by, among other documents, an investigational medicinal product dossier (the Common Technical Document) with supporting information prescribed by Directive 2001/20/EC, Directive 2005/28/EC, where relevant the implementing national provisions of the individual EU Member States and further detailed in applicable guidance documents.
In April 2014, the new Clinical Trials Regulation, (EU) No 536/2014 (Clinical Trials Regulation) was adopted. The Clinical Trials Regulation will be directly applicable in all the EU Member States, repealing the current Clinical Trials Directive 2001/20/EC. Conduct of all clinical trials performed in the European Union will continue to be bound by currently applicable provisions until the new Clinical Trials Regulation becomes applicable. The extent to which ongoing clinical trials will be governed by the Clinical Trials Regulation will depend on when the Clinical Trials Regulation becomes applicable and on the duration of the individual clinical trial. If a clinical trial continues for more than three years from the day on which the Clinical Trials Regulation becomes applicable the Clinical Trials Regulation will at that time begin to apply to the clinical trial. The new Clinical Trials Regulation aims to simplify and streamline the approval of clinical trials in the European Union. The main characteristics of the regulation include: a streamlined application procedure via a single-entry point, the Clinical Trials Information System, or CTIS, a single set of documents to be prepared and submitted for the application as well as simplified reporting procedures for clinical trial sponsors; and a harmonized procedure for the assessment of applications for clinical trials, which is divided in two parts. Part 1 is assessed by the competent authorities of all EU Member States in which an application for authorization of a clinical trial has been submitted (Member States concerned). Part 2 is assessed separately by each Member State concerned. Strict deadlines have been established for the assessment of clinical trial applications. The role of the relevant ethics committees in the assessment procedure will continue to be governed by the national law of the concerned EU Member State. However, overall related timelines will be defined by the Clinical Trials Regulation. On the basis of the results of an independent audit of the CTIS, on April 21, 2021, the EMA Management Board confirmed to the European Commission that the CTIS is fully functional. Based on this, January 31, 2022 was fixed as the date of applicability of the Clinical Trials Regulation (EU) No 536/2014.
The collection and use of personal health data in the European Union, previously governed by the provisions of the Data Protection Directive, is now governed by the General Data Protection Regulation, or the GDPR, which became effective on May 25, 2018. While the Data Protection Directive did not apply to organizations based outside the EU, the GDPR has expanded its reach to include any business, regardless of its location, that provides goods or services to residents in the EU. This expansion would incorporate any clinical trial activities in EU member states. The GDPR imposes strict requirements on controllers and processors of personal data, including special protections for “sensitive information” which includes health and genetic information of data subjects residing in the EU. GDPR grants individuals the opportunity to object to the processing of their personal information, allows them to request deletion of personal information in certain circumstances, and provides the individual with an express right to seek legal remedies in the event the individual believes his or her rights have been violated. Further, the GDPR imposes strict rules on the transfer of personal data out of the European Union to the United States or other regions that have not been deemed to offer “adequate” privacy protections. Failure to comply with the requirements of the GDPR and the related national data protection laws of the European Union Member States, which may deviate slightly from the GDPR, may result in fines of up to 4% of global revenues, or €20,000,000, whichever is greater. As a result of the implementation of the GDPR, we may be required to put in place additional mechanisms ensuring compliance with the new data protection rules.
There is significant uncertainty related to the manner in which data protection authorities will seek to enforce compliance with GDPR. For example, it is not clear if the authorities will conduct random audits of companies doing business in the EU, or if the authorities will wait for complaints to be filed by individuals who claim their rights have been violated. Enforcement uncertainty and the costs associated with ensuring GDPR compliance are onerous and may adversely affect our business, financial condition, results of operations and prospects.
Should we utilize third party distributors, compliance with such foreign governmental regulations would generally be the responsibility of such distributors, who may be independent contractors over whom we have limited control.
Facilities
In July 2023, we signed a lease to move into 2,686 square feet of office space at 1 Enterprise Drive, Suite 430, Shelton, Connecticut (the “Shelton Lease”) to improve recruiting of staff and to reduce costs. The Shelton Lease commenced on September 1, 2023 and has a 5.5 year term. The initial payments for base rent are zero for the first
six months, $2,910 for each of the next six months, and gradually increase to $3,275 per month for the final months of the lease. The Company has an option to cancel this lease after 36 months.
Commercialization
We intend to pursue the complete development to our product candidates and, if marketing approval is obtained, to commercialize our product candidates on our own, or potentially with a partner, in the United States and other regions. We currently have no sales, marketing or commercial product distribution capabilities and have no experience as a company commercializing products. However, if necessary, we intend to hire appropriately to build the necessary infrastructure and capabilities over time for the United States, and potentially other regions, following further advancement of our product candidates. Clinical data, the size of the addressable patient population, the size of the commercial infrastructure and manufacturing needs may all influence or alter our commercialization plans.
Manufacturing
We have established an operations leadership team with extensive experience in manufacturing drugs based on amphiphilic agents, and in the construction, validation, approval and operation of facilities designed to manufacture these products. We have established an operations leadership team with extensive experience in manufacturing of the SHAO and INT230-6 product candidate. Our team has developed a reproducible manufacturing process for SHAO and our product candidates. In 2016 we produced our first batch of INT230-6 under FDA regulated cGMP and have scaled up the product successfully. We generated and continue to generate stability data showing that INT230-6 had acceptable stability through 36 months using validated analytical methods.
Competition
The development and commercialization of new product candidates is highly competitive. We face competition from major pharmaceutical, specialty pharmaceutical and biotechnology companies among others with respect to INT230-6 and will face similar competition with respect to any product candidates that we may seek to develop or commercialize in the future. We compete in pharmaceutical, biotechnology and other related markets that develop immune-oncology therapies for the treatment of cancer. There are other companies working to develop new drugs, immunotherapies and other approaches for the treatment of cancer including divisions of large pharmaceutical and biotechnology companies of various sizes. The large pharmaceutical and biotechnology companies that have commercialized and/or are developing immune-based treatments for cancer include AstraZeneca, Bristol-Myers Squibb, Gilead Sciences, Inc., Merck & Co., Novartis, Pfizer and Genentech, Inc. In addition, other companies have oncology divisions including large companies such as Eli Lilly and GlaxoSmithKline or and several smaller midsize organizations.
Some of the products and therapies developed by our competitors are based on scientific approaches that are the similar to our approach, including with respect to the use of intratumoral delivery or activation of the immune system. Other competitive products and therapies are based on entirely different approaches. We are aware that Oncorus, Inc., Replimune Group, Inc., Amgen Inc., ImmVira Co., Ltd., IconOVir Bio, Inc., and FerGene, Inc., among others, are developing immunotherapies that may have utility for the treatment of indications that we are targeting. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
Many of the companies we compete against or may compete against in the future have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved drugs than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in concentration of even more resources among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel, in establishing clinical trial sites and enrolling subjects for our clinical trials and in acquiring technologies complementary to, or necessary for, our programs.
We could see a reduction or elimination of our commercial opportunity if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, or are more convenient or are less expensive than any products that we or our collaborators may develop. Our competitors also may obtain FDA or foreign regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. The key competitive factors affecting the success of all our product candidates, if approved, are likely to be their efficacy, safety, convenience and
price, if required, the level of biosimilar or generic competition and the availability of reimbursement from government and other third-party payors.
Intellectual Property
We have a robust intellectual property position with 15 issued patents (with 3 of such patents being issued in the US). We have the ability to enforce our patent claims in 38 countries including the U.S. and all external major pharmaceutical markets. Four foreign patent applications are pending.
Our three United States Patent and Trademark Office (PTO) issued patents are as follows; (i) US Patent Number 9,351,997 is directed to a method of treating cancer, with a registration date of May 31, 2016 and an expiration date of December 6, 2033, (ii) US Patent Number 9,636,406 is directed to a method of treating cancer, with a registration date of May 2, 2017 and an expiration date of September 15, 2033, and (iii) US Patent Number 10,888,618 is directed to a method of treating cancer, with a registration date of January 12, 2021 and an expiration date of September 15, 2033.
We also have one U.S. patent application pending. US Patent Application Number 17/108,099 is directed to a method of treating cancer, with a filing date of December 1, 2020. We are prosecuting patents in every major market and have been granted patents in Australia, Canada, China, the 27 European Union countries (national phase filings were made for Austria, Belgium, Cypress, Czech Republic, Denmark, Finland, France, Germany, Greece, Italy, Ireland, Liechtenstein, Luxembourg, Macedonia, Malta, Monaco, the Netherlands, Norway, Poland, Portugal, Romania, San Marino, Singapore, Spain, Sweden, Switzerland, Turkey, and the United Kingdom), Israel, Japan, Macau, Russia, Singapore, South Africa, and South Korea. Patent applications are pending in Brazil, Chile, Mexico and India.
Each application and issued patent has multiple claims directed to technology, methods, formulations and our lead product candidates. Together with trade secrets, know-how and continuing technological innovation, we believe that our IP position is thorough, novel, non-obvious and has been reduced to practice. The technology underlying the pending patent application directed to our lead product candidates has been developed by us and not acquired from in-licensing from any third party.
Employees and Human Capital Resources
As of March 1, 2024, we had seventeen employees and contractors, including one with an M.D. and one with a Ph.D. degree, consisting of two part-time and five full-time and employees, and ten contractors. We have never had a work stoppage, and none of our employees is represented by a labor organization or under any collective-bargaining arrangements. We consider our employee relations to be good.
Employee levels are managed to align with the pace of our business and management believes that it has sufficient human capital to operate its business successfully.
Talent Attraction and Engagement
We seek to foster community, inclusion, and diversity throughout the organization by identifying talent culture adds, with a targeted emphasis on the value and contributions of each employee. Our talent attraction strategy includes utilizing employee referrals and networks, with a generous referral award, job boards, including those focused on diverse talent, and partnerships with organizations representing underrepresented communities. All job descriptions are thoroughly reviewed for consistent and inclusive language. We retain our talent through open and honest communication. Leadership is accessible to all levels of the organization. Feedback is encouraged through formal surveys, regular employee check ins, and the opportunity to provide anonymous suggestions. We continuously seek to improve, and we remain nimble in our ability to implement suggestions that will further benefit our employees. Employees know they have a real opportunity to be heard and to affect our business and culture.
Training and Development
We empower employees to develop their skills and abilities by following our core values and acting on great ideas regardless of their role or function. We work to provide an environment where talented individuals and teams can take control of their career growth. We provide a wide range of learning and development opportunities in both individual and group settings. We have ongoing career growth conversations, beyond a formal review process, and believe in investing in career growth and promoting from within. Similarly, we encourage employees to follow their interests and learn about new roles and departments. Employees can continue their growth by taking on new career trajectories within our growing organization.
Compensation and Benefits
In order to be an employer of choice and maintain the strength of our workforce, we consistently assess the current business environment and labor market to refine our compensation and benefits programs and other resources available.
We offer our employees a holistic total rewards package with premier health and welfare programs for employees and family members. We provide compensation and benefits programs to help meet the needs of our employees and reward their efforts and contributions. We use internal and external resources to help develop plans that are fair and reward our employees’ commitment and performance with the goal of attracting and retaining high performing individuals. Third party survey results show we consistently provide rich benefits, and that our annual merit increase percentages are well above average.
In addition to salaries, we offer dynamic competitive compensation programs that are in line with our peers and industry. To reward employee contributions and enable them to share in the success of the Company, all employees receive generous and attainable incentive compensation beyond their base salary and equity compensation opportunities. We offer a 401(k) with employer match, employer-subsidized insurance benefits which are both robust and cost effective, flexible spending accounts, and employee assistance programs, among many other employee benefits. Recognizing the importance of work/life balance, employees are not limited to a predetermined number of vacation days, and we offer employees an above average number of paid holidays. We offer company paid family leave and all employees receive full incentive compensation during approved leaves of absence.
We maintain pay equity in the U.S. for women and men and people of all races for employees performing similar work.
Health and Wellness
The success of our business is fundamentally connected to the well-being of our people. We strive to provide a work environment where our employees feel safe and are comfortable working and receive support.
Understanding and valuing the importance of work life balance, we have maintained a flexible work from home arrangement, leaving it to employees to determine a schedule that best fits their individual needs. We keep meeting times and deadlines within regular business hours and evaluate workloads to ensure even distribution and balance. For those who choose to come into our offices, we have created spaces that foster social engagement and sponsor reoccurring onsite events. Employee mental health is a top company priority, and we promote dialogue to ensure that employees feel supported. We advise employees to regularly take PTO, facilitate workshops promoting personal well-being, provide extensive subsidized health benefits, including access to mental health resources, and provide for gym reimbursement.
Corporate and Available Information
Our principal executive offices are located at 1 Enterprise Drive, Suite 430, Shelton, CT 06484-4779 and our telephone number is (203) 221-7381. Our website address is www.intensitytherapeutics.com. Our website and the information on, or that can be accessed through our website, will not be deemed to be incorporated by reference into this Annual Report on Form 10-K or any other report we file or furnish to the SEC.
We make available on our website, free of charge, our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and any amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission, or the SEC. Our SEC reports can be accessed through the Investors section of our internet website. Further, a copy of this Annual Report on Form 10-K is located at the SEC’s Public Reference Rooms at 100 F Street, N.E., Washington, D. C. 20549. Information on the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330. The SEC maintains a website that contains reports, proxy and information statements and other information regarding our filings at http://www.sec.gov.
Item 1A. Risk Factors
An investment in our common stock is speculative and involves a high risk, including a risk of your entire investment. You should carefully consider the risks described below and the other information in this Annual Report before buying shares in Intensity Therapeutics, Inc. These are risks and uncertainties that management believes are most likely to be material and therefore are important for an investor to consider. Our business operations and results may also be adversely affected by additional risks and uncertainties not presently known to us, or which are currently deemed immaterial, or which are similar to those faced by other companies in the pharmaceutical industry or business in general.
If any of the following risks or uncertainties actually occurs, our business, financial condition, results of operations, or cash flows would likely suffer. In that event, the value of our stock could decline, perhaps significantly.
Summary of Risk Factors
Investing in our securities involves significant risks. Any of the factors set forth in the section entitled “Risk Factors” may limit our ability to successfully execute our business strategy. You should carefully consider all of the information set forth in this report and, in particular, you should evaluate the specific factors set forth in the section entitled “Risk Factors” in deciding whether to invest in our securities. Some of the principal risks we face include:
•The market price of our Common Stock may be highly volatile, and you could lose all or part of your investment.
•We are a late-stage clinical biotechnology company with a limited operating history and have not generated any revenue to date from product sales.
•Since our inception, we have incurred, and for the foreseeable future anticipate that we will continue to incur, significant operating losses.
•The report of our independent registered public accounting firm for the year ended December 31, 2023 contains a statement with respect to substantial doubt as to our ability to continue as a going concern as a result of recurring losses from operations and negative cash flows.
•If we fail to establish and maintain an effective system of internal control, we may not be able to report our financial results accurately or to prevent fraud, and could harm our reputation and adversely impact the future trading price of our securities.
•We will need to raise substantial additional funding or we will be forced to delay, reduce or eliminate some of our product-development programs or commercialization efforts.
•We are largely dependent upon the success of our new intratumoral technology, which requires additional development and may never receive regulatory approval or be successfully commercialized.
•We have limited experience conducting cancer clinical trials, and we are subject to risks and challenges that may prevent or delay the completion of our up-coming or on-going clinical trials.
•Our prospects for obtaining additional financing are uncertain.
•We have yet to obtain regulatory approval from the FDA, and therefore we are not currently permitted to market products made using our technology in the United States.
•Delays in FDA approval could be costly to us and prevent us from commercializing our product candidates effectively.
•Even if product candidates using our technology obtain approval, we will be subject to additional ongoing regulatory obligations and oversight.
•The FDA approval process is long, expensive and uncertain.
•Our ability to market a product may be limited by the uses that are approved for that product.
•We may be unable to export or sell products in foreign markets, which will limit our sales opportunities.
•We will rely on third parties to conduct preclinical research and any clinical trials.
•Third-party payors may not reimburse for the use of our product candidates or such reimbursement may be inadequate.
•We are dependent on third parties to manufacture components of the final drug products made using our technology.
•We purchase components for our product candidates from third parties, some of which may be sole-source suppliers.
•We have not entered into long term manufacturing and supply agreements with any producers.
•We have limited experience and may not be successful in commercializing products that use our technology.
•Our plan to use collaborative arrangements with third parties to help finance and to market and sell products using our technology may not be successful.
•We will be dependent on healthcare professionals’ efforts to learn about our product candidates.
•We may need to establish clinical training and centers of excellence to educate and train physicians and healthcare payors, but the key opinion thought leadership required for initial market acceptance within the healthcare arena may take time to develop.
•Rapid technological developments in treatment methods for cancer and competition with other forms of cancer treatments could affect our ability to achieve meaningful revenues or profit.
•Our success depends in part on our ability to obtain patents, maintain trade secret protection, operate without infringing on the proprietary rights of third parties, and commercialize our technology prior to the expiration of our patent protection.
•We may be unable to protect our intellectual property rights because of our limited resources.
•We may be the subject of product liability claims or product recalls.
Risks Related to Our Business, Financial, and Investment Conditions
The market price of our Common Stock may be highly volatile, and you could lose all or part of your investment.
The trading price of our Common Stock is likely to be volatile. We have a relatively small public float due to the ownership percentage of our executive officers, directors and greater than 5% stockholders. As a result of our small public float, our Common Stock may be less liquid and have greater stock price volatility than the common stock of companies with broader public ownership.
Our stock price could be subject to wide fluctuations in response to a variety of other factors, which include:
•whether we achieve our anticipated corporate objectives;
•changes in financial or operational estimates or projections;
•termination of the lock-up agreements or other restrictions on the ability of our stockholders and other security holders to sell shares; and
•general economic or political conditions in the United States or elsewhere.
In addition, the stock market in general, and the stock of clinical-stage biotechnology companies in particular, has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Such rapid and substantial price volatility, including any stock run-up, may be unrelated to our actual or expected operating performance and financial condition or prospects, making it difficult for prospective investors to assess the rapidly changing value of our stock. This volatility may prevent you from being able to sell your securities at or above the price you paid for your securities.
We are a late-stage clinical biotechnology company with a limited operating history and have not generated any revenue to date from product sales.
We are a late-stage clinical, pre-commercial company with only a limited operating history upon which to base an evaluation of our current business and future prospects and how we will respond to competitive, financial or technological challenges. Biotechnology product development is a highly speculative undertaking and involves a substantial degree of risk. We were incorporated under the laws of the State of Delaware in November 2012. Since inception, we have focused substantially all of our efforts and financial resources on raising capital and developing our initial product candidates. We have no products approved for commercial sale and therefore have never generated any revenue from product sales, and we do not expect to do so in the foreseeable future. We have not obtained regulatory approvals for any of our product candidates. Consequently, the revenue-generating potential of our business is unproven and uncertain. Even if our product candidates receive regulatory approval, we may be unable to successfully introduce and market them at prices that would permit us to operate profitably.
We have incurred significant operating losses since our inception and anticipate that we will incur continued losses for the foreseeable future.
To date, we have financed our operations primarily through an initial investment from our founder and the issuance and sale of Common Stock, our convertible preferred stock and convertible debt notes, to outside investors in private equity financings. In July 2023, we also received the proceeds from our initial public offering (“IPO”). From our inception through December 31, 2023, we raised an aggregate of $54.5 million in cash received from the net proceeds from such transactions. As of December 31, 2023, our cash and cash equivalents and investments were $14.8 million. We have incurred net losses in each year since our inception, and we had an accumulated deficit of $50.5 million as of December 31, 2023. For the years ended December 31, 2023 and 2022, we reported net losses attributable to stockholders of $11.9 million and $7.6 million, respectively.
We expect to continue to incur significant expenses and operating losses over the next several years and for the foreseeable future. Substantially all of our operating losses have resulted from costs incurred in connection with our research and development programs and from general and administrative costs associated with our operations. We expect our research and development expenses to significantly increase in connection with the commencement and continuation of clinical trials of our product candidates. In addition, if we obtain marketing approval for our product candidates, we will incur significant sales, marketing and manufacturing expenses. As we are a public company, we will incur additional costs associated with operating as a public company. As a result, we expect to continue to incur significant and increasing operating losses for the foreseeable future. Because of the numerous risks and uncertainties associated with developing biotechnology products, we are unable to predict the extent of any future losses or when we will become profitable, if at all. Even if we do become profitable, we may not be able to sustain or increase our profitability on a quarterly or annual basis. Our prior losses, combined with expected future losses, have had and will continue to have an adverse effect on our stockholders’ deficit and working capital.
The report by our auditors includes a paragraph that states that substantial doubt exists about the Company’s ability to continue as a going concern.
The report of our independent registered public accounting firm for the year ended December 31, 2023 included herein contains an explanatory paragraph concurring with management’s assessment indicating that there is substantial doubt as to our ability to continue as a going concern as a result of recurring losses from operations and negative cash flows. We do not have a history of earnings and, as a result, substantial doubt exists about our ability to continue as a going concern. Further, based on the cash, cash equivalents and marketable debt securities as of December 31, 2023, we only have sufficient cash to continue with our business plan through the end of the first quarter in 2025.
Our continued operations are dependent on our ability to complete equity or debt financings or generate profitable operations. Such financings may not be available or may not be available on reasonable terms. Our financial statements do not include any adjustments that may result from the outcome of this uncertainty. If we are unable to obtain adequate funding in the future, or if we are unable to generate revenue to achieve and sustain profitability, we may not be able to continue as a going concern. We believe that there is substantial doubt as to whether we can raise sufficient funding in order for us to continue operations.
If we fail to establish and maintain an effective system of internal control, we may not be able to report our financial results accurately or to prevent fraud. Any inability to report and file our financial results accurately and timely could harm our reputation and adversely impact the future trading price of our securities.
Effective internal control is necessary for us to provide reliable financial reports and prevent fraud. However, because of our limited resources, there are limited controls over information processing. We have material weaknesses due to (i) a lack of segregation of duties due to limited administrative staff, (ii) limited reconciliation and review procedures over clinical contract accruals as we have rapidly expanded into new, late-stage clinical studies, and (iii) information technology matters regarding user access that aggregate to a material weakness. Our management is composed of a small number of individuals resulting in limitations on segregation of duties. We have focused our segregation of duties to ensure that the actual payments are performed separately from the accounting staff, and the Chief Executive Officer performs a robust review of the financial statements on a monthly basis. All accounting entries and the creation of financial statements, however, have been performed by our Principal Accounting Officer. To address our segregation of duties concerns, in August 2021, we hired a consultant with Danforth Advisors LLC, a company that provides strategic and operational finance and accounting services to life science companies, as interim Chief Financial Officer to add a layer of supervision and control. In addition, in December 2023 we hired a full time CFO who will add an additional layer of segregation of duties within our accounting and payment processing procedures, as well as establishing formal reconciliation review oversight. We will continue to evaluate our internal controls environment to establish a proper control environment, and
plan to implement additional controls over segregation of duties, reconciliation and review procedures, and information technology access controls in the first half of 2024.
Our small size and internal control deficiencies may adversely affect our financial condition, results of operation and access to capital. If we cannot provide reliable financial reports or prevent fraud, we may not be able to manage our business as effectively as we would if an effective control environment existed, and our business and reputation with investors may be harmed.
We will need to raise substantial additional funding. If we are unable to raise capital when needed, we would be forced to delay, reduce or eliminate some or all of our product development programs or commercialization efforts.
The development of biotechnology products is capital-intensive and we expect our expenses to significantly increase in connection with our ongoing activities, particularly as we continue our ongoing clinical trials or initiate future trials and pursue the research and development of, and seek marketing approval for, our product candidates. Our future capital requirements will depend on and could increase significantly as a result of many factors, including:
•our research and product development programs, including clinical studies;
•the timing and costs of our various U.S. and foreign regulatory filings, obtaining approvals, and complying with regulations;
•the timing and costs associated with developing manufacturing operations;
•the timing of product commercialization activities, including marketing and distribution arrangements;
•the timing and costs involved in preparing, filing, prosecuting, defending, and enforcing intellectual property rights; and
•the impact of competing technological and market developments.
We expect that existing cash and cash equivalents and investments will be sufficient to fund our operations and capital expenditure requirements for approximately the next 12 months. Accordingly, we will need to obtain substantial additional funding to continue our operations. We cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of our stockholders and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our shares to decline. The sale of additional equity or convertible securities would dilute all of our stockholders. The incurrence of indebtedness would result in increased fixed payment obligations and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborators or otherwise at an earlier stage than otherwise would be desirable and we may be required to relinquish rights to some of our technologies or product candidates or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects. Any additional fundraising efforts may also divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates.
If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce or eliminate certain of our research and development programs or future commercialization efforts, and may be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially affect our business, financial condition and results of operations.
We are largely dependent upon the success of our new intratumoral technology, which will require additional development before we may be able to seek regulatory approval and may never receive regulatory approval or be successfully commercialized.
The Intensity Therapeutics Technology, a platform for the creation of products to improve treatment of cancer patients, is our only technology. Our entire focus has been on developing, commercializing, and ultimately obtaining regulatory authorizations and approvals of product candidates using this technology. We have invested, and we expect to continue to invest, significant efforts and financial resources in its development. Our ability to generate meaningful revenue, which may not occur for the foreseeable future, if ever, will depend heavily on the successful development, regulatory approval and commercialization of our technology. If we are unable to develop the Intensity Therapeutics Technology, obtain regulatory approval, and sell products using the technology, we will not generate operating revenue or become profitable, and we may be forced to terminate or cease operations.
We have limited experience conducting cancer clinical trials, and we are subject to risks and challenges that may prevent or delay the completion of our upcoming or on-going clinical trials.
We have completed two clinical trials in cancer with 110 patients in metastatic disease and 91 patients in presurgical patients without treatment options. The completed study was a multi-cohort clinical trial testing our product candidate alone or combined with Keytruda® or with Yervoy®. The other study was a randomized Phase 2 study in presurgical breast cancer. Approximately 200 patients have been enrolled in our clinical trials as of March 1, 2024. There will not be any additional enrollment in the first two studies. We have not demonstrated any survival benefit compared to an active control group in a statistically significant and meaningful manner. We have not demonstrated sufficient safety of any product candidate for FDA approval for a given cancer type. Our largest dose on any given day so far has been 244mL containing 122 mg of cisplatin and 24.4 mg of vinblastine sulfate. While these doses are larger than most intravenous doses, we have no indication that higher doses or any dose will be safe or effective. At this time, we do not intend to dose higher in a treatment session than 175mL.
We intend to conduct clinical trials for sarcoma and breast cancer indications, and it may take several years to complete the testing of our product candidates and technology for the indications for which we wish to obtain approval. Failure or delay can occur at any stage of development, for many reasons, including:
•any pre-clinical or clinical test may fail to produce results satisfactory to the FDA or foreign regulatory authorities and preclude us from testing in humans;
•pre-clinical or clinical data can be interpreted in different ways, which could delay, limit, or prevent regulatory approval;
•negative or inconclusive results from a pre-clinical study or clinical trial or adverse medical events during a clinical trial could cause a pre-clinical study or clinical trial to be repeated or a program to be terminated, even if other studies or trials relating to the program are successful;
•the FDA or foreign regulatory authorities can place a clinical hold on a trial if, among other reasons, it finds that patients enrolled in the trial are or would be exposed to an unreasonable and significant risk of illness or injury;
•changes in regulatory agency policies during the period in which we are developing a system, or the period required for review of any application for regulatory agency approval;
•our clinical trials may not demonstrate the safety and efficacy of any system or result in marketable products;
•the FDA or foreign regulatory authorities may request additional clinical trials, including more than one Phase 3 trial, relating to any potential NDA submissions;
•the FDA or foreign regulatory authorities may change their approval policies or adopt new regulations that may negatively affect or delay our ability to bring a system to market or require additional clinical trials; and
•a system may not be approved for all the requested indications.
We face significant competition from other biotechnology and pharmaceutical companies, and our operating results will suffer if we fail to compete effectively.
The biopharmaceutical industry is characterized by intense competition and rapid innovation. We face competition from major pharmaceutical, specialty pharmaceutical and biotechnology companies among others with respect to INT230-6 and will face similar competition with respect to any product candidates that we may seek to develop or commercialize in the future. We compete in pharmaceutical, biotechnology and other related markets that develop immune-oncology therapies for the treatment of cancer. There are other companies working to develop new drugs, immunotherapies and other approaches for the treatment of cancer including divisions of large pharmaceutical and biotechnology companies of various sizes. Many of our competitors have substantially greater financial, technical and other resources, such as larger research and development staff and experienced marketing and manufacturing organizations and well-established sales forces. Smaller or early-stage companies may also prove to be significant competitors, particularly as they develop novel approaches to treating disease indications that our product candidates are also focused on treating. Established pharmaceutical companies may also invest heavily to accelerate discovery and development of novel therapeutics or to in-license novel therapeutics that could make the product candidates that we develop obsolete. Mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated in our competitors. Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in these industries. Our competitors, either alone or with collaborative partners, may succeed in developing, acquiring or licensing on an exclusive basis drug or biologic products that are more effective, safer,
more easily commercialized or less costly than our product candidates or may develop proprietary technologies or secure patent protection that we may need for the development of our technologies and products. We believe the key competitive factors that will affect the development and commercial success of our product candidates are efficacy, safety, tolerability, reliability, convenience of use, price and reimbursement.
There are a number of companies trying to develop intratumoral therapies. However, most of our competitors are currently focused on intratumoral treatment approaches that stimulate immune cells to achieve inflammation rather than directly killing a tumor. This shift to a pure immune-oncology (IO) treatment has reopened the investigations into intratumoral approaches focusing on activating local immune response. Amgen markets a novel genetically modified oncolytic viral-based immunotherapeutic, talimogene laherparepvec (T-Vec), that has been approved for IT use in cutaneous melanoma. While T-Vec is approved solely for local treatment of localized cutaneous melanoma, the drug has not been shown to improve overall survival or have any effect on distal metastases, which will be a critical factor to broader use. Another viral based system is being developed by Replimune. RP1 is Replimune’s genetically modified herpes simplex type 1 virus that is designed to directly destroy tumors and to generate an anti-tumor immune response. This product is being evaluated in a Phase ½, open label, multicenter, dose escalation and expansion, first-in-human (FIH) clinical study to evaluate the safety and tolerability, biodistribution, shedding, and preliminary efficacy of RP1 alone and in combination with nivolumab in adult subjects with advanced and/or refractory solid tumors. The IGNYTE Study, which started in 2017, includes a dose escalation Phase for single agent RP1, an expansion Phase with a combination of RP1 and nivolumab and a Phase 2 portion in specified tumor types for the combination therapy. Dose escalation of RP1 by intratumoral injection in superficial tumors and in visceral tumors. The objective of this viral approach is to transfect the granulocyte-macrophage colony-stimulating factor gene into the tumor microenvironment to recruit a local inflammatory response that would promote a systemic immune response.
A number of high-profile neoadjuvant immunotherapy trials are currently underway. Several studies listed on the ClinicalTrials.gov website, are studying a diverse array of immune modulating therapies in the neoadjuvant setting for treatment of solid tumors. Recent and ongoing clinical trials utilizing neoadjuvant intratumoral immunotherapy include intratumoral agents such as:
•Poly-ICLC (Hiltonol) for prostate cancer in phase 1 (NCT03262103), which is recruiting,
•TLR7 agonist (Imiquimod) for treatment of melanoma in phase 3 (NCT01720407), which is active though not yet recruiting,
•TLR9 agonist (CMP-001) with anti-PD-1 (nivolumab) for melanoma and lymph node cancer in phase 2 (NCT0361864), which is recruiting, and
•TLR8 agonist (VTX-2337) with anti-PD-1 (Tislelizumab) for head and neck cancer in phase 1 (NCT03906526), and not yet recruiting.
Other local treatment approaches that had been explored by companies such as Merck also attempt to recruit the immune system cells into the local tumor microenvironment with intratumoral delivery of other agents. Data on several other intratumorally-delivered agents such as STING agonists, RIG-1, and TLR9 have been presented at major cancer conferences.
Our belief is that our competitors have formulated their products without consideration of the inability of water-based products to be well absorbed into a tumor’s lipophilic, high-pressure microenvironment. Attempts at the stimulation of an inflammatory response or efforts to attract immune cells into a hostile live, rapidly growing tumor still pose a number of challenges. Accordingly, there remains a continued unmet need for the development of direct IT therapies for solid tumors that provide high local killing efficacy coupled with nontoxic systemic anti-cancer effects. We believe we have created a product candidate having the necessary physical and chemical properties to overcome the local delivery challenges. Evidence shows the mechanism of tumor killing achieved by our drug candidate also leads to systemic immune activation in certain cancers.
We anticipate competing with other companies that are focused on treating disease indications that our product candidates are also focused on treating. A competitor may develop technologies focused on the same disease pathway as our technology or may focus on treating the targeted disease in a completely different manner. To the extent a new drug is developed that is more efficacious than any product candidate developed by us, this could reduce or negate the need for our product candidate. In addition, while we believe our product candidates may be used in conjunction with existing or emerging SOC in certain disease indications, as companies continue to improve upon existing SOC, more efficacious drug therapies could become available, reducing or completely negating the benefit of our product candidates. Our competitors may also include companies that are or will be developing therapies for the same therapeutic areas that we are targeting within our early pipeline.
Even if we are successful in achieving regulatory approval to commercialize a product candidate ahead of our competitors, our future pharmaceutical products may face direct competition from generic and other follow-on drug products. Any of our product candidates that may achieve regulatory approval in the future may face competition from generic products earlier or more aggressively than anticipated, depending upon how well such approved products perform in the U.S. prescription drug market. Our ability to compete also may be affected in many cases by insurers or other third-party payors seeking to encourage the use of generic products. Generic products are expected to become available over the coming years. Even if our product candidates achieve marketing approval, they may be priced at a significant premium over competitive generic products, if any have been approved by then.
In addition to creating the 505(b)(2) NDA pathway, the Hatch-Waxman Amendments to the federal Food, Drug, and Cosmetic Act (FDCA) authorized the FDA to approve generic drugs that are the same as drugs previously approved for marketing under the NDA provisions of the statute pursuant to ANDAs. An ANDA relies on the preclinical and clinical testing conducted for a previously approved reference listed drug (“RLD”), and must demonstrate to the FDA that the generic drug product is identical to the RLD with respect to the active ingredients, the route of administration, the dosage form, and the strength of the drug and also that it is “bioequivalent” to the RLD. The FDA is prohibited by statute from approving an ANDA when certain marketing or data exclusivity protections apply to the RLD. If any such competitor or third party is able to demonstrate bioequivalence without infringing our patents, then this competitor or third party may then be able to introduce a competing generic product onto the market.
We cannot predict the interest of potential follow-on competitors or how quickly others may seek to come to market with competing products, whether approved as a direct ANDA competitor or as a 505(b)(2) NDA referencing one of our future drug products. If the FDA approves generic versions of our drug candidates in the future, should they be approved for commercial marketing, such competitive products may be able to immediately compete with us in each indication for which our product candidates may have received approval, which could negatively impact our future revenue, profitability and cash flows and substantially limit our ability to obtain a return on our investments in those product candidates.
Even if we obtain regulatory approval of our product candidates, the availability and price of our competitors’ products could limit the demand and the price we are able to charge for our product candidates. We may not be able to implement our business plan if the acceptance of our product candidates is inhibited by price competition or the reluctance of physicians to switch from existing methods of treatment to our product candidates, or if physicians switch to other new drug or biologic products or choose to reserve our product candidates for use in limited circumstances. For additional information regarding our competition, see “Business — Competition.”
Our prospects for obtaining additional financing, as needed, are uncertain and our failure to obtain needed financing could affect our ability to pursue future growth.
We will need to raise additional funds in the future to develop or enhance our product candidates, to fund expansion, to conduct additional clinical trials and to fund general operating expenses. For example, with regard to our Phase 3 sarcoma study (IT-03) and Phase 2/3 early-stage breast cancer study (IT-04), we expect that our cash and cash equivalents and investments will be sufficient to allow us to obtain regulatory authorizations to proceed for these trials. There is no assurance that additional financing will be available on terms favorable to us, or at all. If additional funds are raised through the issuance of equity or convertible debt securities, the percentage ownership of our stockholders would be reduced, and these securities might have rights, preferences, or privileges senior to those of our current stockholders. If adequate funds are not available on acceptable terms, our ability to fund our expansion, take advantage of unanticipated opportunities, develop or enhance services or products, or otherwise respond to competitive pressures would be significantly limited.
Inadequate funding for the FDA, the SEC and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which our operations may rely, including those that fund research and development activities, is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new products to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical employees and stop critical activities.
Separately, the FDA has announced its commitment to achieving timely reviews of applications for medical products during the COVID-19 pandemic in line with its user fee performance goals; however, the FDA may not be able to continue its current pace and review timelines could be extended, including where a pre-approval inspection or an inspection of clinical sites is required and due to the COVID-19 pandemic and travel restrictions the FDA is unable to complete such required inspections during the review period. On March 10, 2020, the FDA announced its intention to postpone most inspections of foreign manufacturing facilities, and on March 18, 2020, the FDA temporarily postponed routine surveillance inspections of domestic manufacturing facilities. Subsequently, on July 10, 2020, the FDA announced its intention to resume certain on-site inspections of domestic manufacturing facilities subject to a risk-based prioritization system. The FDA intends to use this risk-based assessment system to identify the categories of regulatory activity that can occur within a given geographic area, ranging from mission critical inspections to resumption of all regulatory activities. Additionally, on April 15, 2021, the FDA issued a guidance document in which the FDA described its plans to conduct voluntary remote interactive evaluations of certain drug manufacturing facilities and clinical research sites. According to the guidance, the FDA intends to request such remote interactive evaluations in situations where an in-person inspection would not be prioritized or deemed mission-critical, or where direct inspection is otherwise limited by travel restrictions, but where the FDA determines that remote evaluation would still be appropriate. Regulatory authorities outside the U.S. may adopt similar restrictions or other policy measures in response to the COVID-19 pandemic and may experience delays in their regulatory activities. If a prolonged government shutdown occurs, or if global health concerns continue to prevent the FDA or other regulatory authorities from conducting their regular inspections, reviews, or other regulatory activities, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, in our operations as a public company, future government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
Risks Related to FDA and Foreign Regulatory Approval
Clinical development involves a lengthy, complex and expensive process, with an uncertain outcome, and the results of preclinical studies and early-stage clinical trials of our product candidates may not be predictive of the results of later-stage clinical trials.
The development and approval process in the United States may take many years, require substantial resources, and may never lead to the approval of any of our product candidates by the FDA for use in the United States. To obtain the requisite regulatory approvals to commercialize any product candidates, we must demonstrate through extensive preclinical studies and clinical trials that our product candidates are safe and effective in humans. Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. In particular, the general approach for FDA approval of a new drug is dispositive data from one or two adequate and well-controlled, Phase 3 clinical trials of the relevant drug in the relevant patient population. Phase 3 clinical trials typically involve hundreds of patients, have significant costs and take years to complete. A product candidate can fail at any stage of testing, even after observing promising signals of activity in earlier preclinical studies or clinical trials. The results of preclinical studies and early clinical trials of our product candidates may not be predictive of the results of later-stage clinical trials. In addition, initial success in clinical trials may not be indicative of results obtained when such trials are completed. There is typically an extremely high rate of attrition from the failure of product candidates proceeding through clinical trials. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy profile despite having progressed through preclinical studies and initial clinical trials. A number of companies in the biotechnology and biopharmaceutical industries have suffered significant setbacks in advanced clinical trials due to lack of efficacy or unacceptable safety issues, notwithstanding promising results in earlier trials. Most product candidates that commence clinical trials are never approved as therapeutic products, and there can be no assurance that any of our future clinical trials will ultimately be successful or support further clinical development of INT230-6 or any of our other product candidates. Product candidates that appear promising in the early phases of development may fail to reach the market for several reasons, including:
•preclinical studies or clinical trials may show the product candidates to be less effective than expected (e.g., a clinical trial could fail to meet its primary endpoint(s)) or to have unacceptable side effects or toxicities;
•failure to establish clinical endpoints that applicable regulatory authorities would consider clinically meaningful;
•failure to receive the necessary regulatory approvals;
•manufacturing costs, formulation issues, pricing or reimbursement issues, or other factors that make a product candidate uneconomical; and
•the proprietary rights of others and their competing products and technologies that may prevent one of our product candidates from being commercialized.
In addition, differences in trial design between early-stage clinical trials and later-stage clinical trials make it difficult to extrapolate the results of earlier clinical trials to later clinical trials. Moreover, clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in clinical trials have nonetheless failed to obtain marketing approval of their products.
Additionally, we expect that some of our trials will be open-label studies, where both the patient and investigator know whether the patient is receiving the investigational product candidate as a monotherapy or in combination with an existing approved drug. Most typically, open-label clinical trials test only the investigational product candidate and sometimes do so at different dose levels. Open-label clinical trials are subject to various limitations that may exaggerate any therapeutic effect as patients in open-label clinical trials are aware when they are receiving treatment. In addition, open-label clinical trials may be subject to an “investigator bias” where those assessing and reviewing the physiological outcomes of the clinical trials are aware of which patients have received treatment and may interpret the information of the treated group more favorably given this knowledge. Therefore, it is possible that positive results observed in open-label trials will not be replicated in later placebo-controlled trials.
In addition, the standards that the FDA and comparable foreign regulatory authorities use when regulating our product candidates require judgment and can change, which makes it difficult to predict with certainty how they will be applied. Although we are initially focusing our efforts on development of small-molecule drug products, we may in the future pursue development of biological products, which could make us subject to additional regulatory requirements. Any analysis we perform of data from preclinical and clinical activities is subject to confirmation and interpretation by regulatory authorities, which could delay, limit or prevent regulatory approval. We may also encounter unexpected delays or increased costs due to new government regulations. Examples of such regulations include future legislation or administrative action, or changes in FDA policy during the period of product development and FDA regulatory review. We cannot predict whether legislative changes will be enacted, or whether FDA or foreign regulations, guidance or interpretations will be changed, or what the impact of such changes, if any, may be. The FDA may also require a panel of experts, referred to as an Advisory Committee, to deliberate on the adequacy of the safety and efficacy data to support approval. The opinion of the Advisory Committee, although not binding, may have a significant impact on our ability to obtain approval of any product candidates that we develop.
We may seek to conduct clinical trials in foreign countries, as well as in the United States. If we continue to seek to conduct clinical trials in foreign countries or pursue marketing approvals in foreign jurisdictions, we must comply with numerous foreign regulatory requirements governing, among other things, the conduct of clinical trials, manufacturing and marketing authorization, pricing and third-party reimbursement. The foreign regulatory approval process varies among countries and may include all of the risks associated with FDA approval described above as well as risks attributable to the satisfaction of local regulations in foreign jurisdictions. Moreover, the time required to obtain approval from foreign regulatory agencies may differ from that required to obtain FDA approval. Approval by the FDA does not ensure approval by regulatory authorities outside the United States and vice versa.
Successful completion of clinical trials is a prerequisite to submitting a marketing application to the FDA and similar marketing applications to comparable foreign regulatory authorities, for each product candidate and, consequently, the ultimate approval and commercial marketing of any product candidates. We may experience negative or inconclusive results, which may result in our deciding, or our being required by regulators, to conduct additional clinical studies or trials or abandon some or all of our product development programs, which could have a material adverse effect on our business.
We will likely need separate regulatory approvals for every therapeutic agent or combination of compounds that we intend to develop and market using our technology.
Although many drugs have been approved by the FDA for use as therapeutic agents, regulatory approval is likely required in the United States for the combined enhancer component with the drug component(s) and the specific indication, dose, and route of administration of the therapeutic agent or agents used in our system.
We will likely need to obtain separate regulatory approvals for products using our technology with every therapeutic agent or combination of compounds used with our system that we intend to market. All the manufacturing facilities used to manufacture components or assemble our system must be inspected and meet legal requirements. Securing regulatory
approval requires the submission of extensive pre-clinical and clinical data and other supporting information for each proposed therapeutic indication to establish to the FDA’s satisfaction the product’s safety, efficacy, potency, and purity for each intended use. The pre-clinical testing and clinical trials of any products using our technology with any therapeutic agent or compound we use must comply with the regulations of the FDA and other federal, state, and local government authorities in the United States. Clinical development is a long, expensive, and uncertain process and is subject to delays. We may encounter delays or rejections for various reasons, including our inability to enroll enough patients to complete our clinical trials. Moreover, approval policies or regulations may change. If we do not obtain and maintain regulatory approval for our system and our use of therapeutic agents, our results of operations will be harmed.
Failure to obtain, or delay in obtaining, regulatory approvals would likely have a material adverse effect on our business, financial condition and results of operations.
During its development, our product candidates and technology will be subject to extensive and rigorous government regulation by the FDA and possibly other foreign regulatory agencies. The FDA regulates the research, development, pre-clinical and clinical testing, manufacture, safety, effectiveness, record keeping, reporting, labeling, storage, approval, advertising, promotion, sale, distribution, import, and export of pharmaceutical and medical device products. Failure to comply with FDA and other applicable regulatory requirements, either before or after product approval, may subject us to administrative or judicially imposed sanctions.
We are not permitted to market products made using our technology in the United States unless and until we obtain regulatory approval from the FDA.
To market the product candidate in the United States, we must submit to the FDA and obtain FDA approval of an NDA. An IND application is the first step in the regulatory process. Under an IND, a Company develops a drug in the hopes of someday submitting to the FDA the NDA to permit marketing of the drug. An NDA must be supported by extensive clinical and preclinical data, as well as extensive information regarding CMC to demonstrate the safety and effectiveness of the applicable product candidate. Regulatory approval of an NDA is not guaranteed. The number and types of preclinical studies and clinical trials that will be required varies depending on the product candidate, the disease or condition that the product candidate is designed to target, and the regulations applicable to any product candidate. Despite the time and expense associated with preclinical and clinical studies, failure can occur at any stage and we could encounter problems that cause us to repeat or perform additional preclinical studies, CMC studies or clinical trials. The FDA and similar foreign authorities could delay, limit or deny approval of a product candidate for many reasons, including because they:
•may not deem a product candidate to be adequately safe and effective;
•may not find the data from preclinical studies, CMC studies, and clinical trials to be sufficient to support a claim of safety and efficacy;
•may interpret data from preclinical studies, CMC studies, and clinical trials significantly differently than we do;
•may not approve the manufacturing processes or facilities associated with our product candidates;
•may change approval policies (including with respect to our product candidates’ class of drugs) or adopt new regulations; or
•may not accept a submission due to, among other reasons, the content or formatting of the submission.
Delays in FDA approval could be costly to us and prevent us from commercializing our product candidates effectively.
The regulatory review and approval process is lengthy, expensive, and inherently uncertain. As part of the U.S. Prescription Drug User Fee Act, the FDA has a goal to review and act on a percentage of all submissions in a given time frame. The general review goal for a drug application is ten to twelve months for a standard application and six months for a priority review application. The FDA’s review goals are subject to change and it is unknown whether the review of an NDA filing for any of our product candidates will be completed within the FDA’s review goals or will be delayed. Moreover, the duration of the FDA’s review may depend on the number and types of other NDAs that are submitted to the FDA around the same time. The development and approval process may take many years, require substantial resources, and may never lead to the approval of a product. Failure to obtain or delays in obtaining regulatory approvals may:
•adversely affect the commercialization of our current technology or any products that we develop in the future;
•impose additional costs on us;
•diminish any competitive advantages that may be attained; and
•adversely affect our ability to generate revenues.
We have received one Fast Track Designation, and may continue to seek Breakthrough Therapy Designations or other Fast Track Designations from the FDA, for certain of our product candidates in certain indications, but receipt of either such designation may not actually lead to a faster development or regulatory review or approval process.
In 2018, we received Fast Track Designation by the FDA to use INT230-6 in metastatic triple negative breast cancer for patients whose cancer has progressed following one or two prior drug treatments. We may continue to seek Breakthrough Therapy Designation or Fast Track Designation for our product candidates or for other indications.
A breakthrough therapy is defined as a product that is intended, alone or in combination with one or more other products, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. For products that have been designated as breakthrough therapies, interaction and communication between the FDA and the sponsor of the trial can help to identify the most efficient path for clinical development while minimizing the number of patients placed in ineffective control regimens. Products designated as breakthrough therapies by the FDA can also be eligible for accelerated approval.
Designation as a breakthrough therapy is within the discretion of the FDA. Accordingly, even if we believe one of our product candidates meets the criteria for designation as a breakthrough therapy, the FDA may disagree and instead determine not to make such designation. In any event, the receipt of a Breakthrough Therapy Designation for a product candidate may not result in a faster development process, review or approval compared to products considered for approval under conventional FDA procedures and does not assure ultimate approval by the FDA. In addition, even if one or more of our product candidates qualify as breakthrough therapies, the FDA may later decide that the products no longer meet the conditions for qualification and rescind the breakthrough designation.
If a product is intended for the treatment of a serious or life-threatening condition and the product demonstrates the potential to address unmet medical needs for this condition, the product sponsor may apply for Fast Track Designation. The FDA has broad discretion whether or not to grant this designation, so even if we believe a particular product candidate is eligible for this designation, we cannot assure you that the FDA would decide to grant it. Even though we have received Fast Track Designation to use INT230-6 in certain indications, or if we receive Fast Track Designation for other drug products or indications, we may not experience a faster development process, review or approval compared to conventional FDA procedures. The FDA may withdraw Fast Track Designation if it believes that the designation is no longer supported by data from our clinical development program.
If we encounter difficulties enrolling patients in our clinical trials, our clinical development activities could be delayed or otherwise adversely affected.
We may experience difficulties in patient enrollment in our clinical trials for a variety of reasons. The timely completion of clinical trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the trial until its conclusion. The enrollment of patients depends on many factors, including:
•the patient eligibility and exclusion criteria defined in the protocol;
•the size of the patient population required for analysis of the trial’s primary endpoints;
•the proximity of patients to trial sites;
•the design of the trial;
•our ability to recruit clinical trial investigators with the appropriate competencies and experience;
•our ability to obtain and maintain patient consents; and
•the risk that patients enrolled in clinical trials will drop out of the trials before completion.
In addition, our clinical trials will compete with other clinical trials for product candidates that are in the same therapeutic areas as our product candidates, and this competition will reduce the number and types of patients available to us, because some patients who might have opted to enroll in our trials may instead opt to enroll in a trial being conducted by one of our competitors. Since the number of qualified clinical investigators is limited, we expect to conduct some of our
clinical trials at the same clinical trial sites that some of our competitors use, which will reduce the number of patients who are available for our clinical trials in such clinical trial site.
Delays in patient enrollment may result in increased costs or may affect the timing or outcome of our future clinical trials, which could prevent completion of these trials and adversely affect our ability to advance the development of our product candidates.
We will rely on third parties to conduct certain of the preclinical research and any clinical trials for products using our technology, and if those third parties perform in an unsatisfactory manner, it may harm our business.
We do not currently have the ability to independently conduct any clinical trials. We intend to rely on CROs and clinical trial sites to ensure the proper and timely conduct of our preclinical studies and clinical trials, and we expect to have limited influence over their actual performance. We rely upon CROs to monitor and manage data for our clinical programs, as well as the execution of future preclinical studies. We expect to control only certain aspects of our CROs’ activities. Nevertheless, we will be responsible for ensuring that each of our preclinical studies and clinical trials is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our reliance on the CROs does not relieve us of our regulatory responsibilities.
We and our CROs are required to comply with the good laboratory practices, or GLPs, and GCPs, which are regulations and guidelines enforced by the FDA and comparable foreign regulatory authorities in the form of International Conference on Harmonization guidelines for any of our product candidates that are in preclinical and clinical development. The regulatory authorities enforce GCPs through periodic inspections of trial sponsors, principal investigators and clinical trial sites. Although we rely on CROs to conduct GCP-compliant clinical trials, we remain responsible for ensuring that each of our GLP preclinical studies and clinical trials is conducted in accordance with its investigational plan and protocol and applicable laws and regulations. If we or our CROs fail to comply with GCPs, the clinical data generated in our clinical trials may be deemed unreliable, and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. Accordingly, if our CROs fail to comply with these regulations or fail to recruit a sufficient number of subjects, we may be required to repeat clinical trials, which would delay the regulatory approval process.
Our reliance on third parties to conduct clinical trials will result in less direct control over the management of data developed through clinical trials than would be the case if we were relying entirely upon our own staff. Communicating with CROs and other third parties can be challenging, potentially leading to mistakes as well as difficulties in coordinating activities. Such parties may:
•have staffing difficulties;
•fail to comply with contractual obligations;
•experience regulatory compliance issues; or
•undergo changes in priorities or become financially distressed.
These factors may adversely affect the willingness or ability of third parties to conduct our clinical trials and may subject us to unexpected cost increases that are beyond our control. If our CROs do not successfully carry out their contractual duties or obligations, fail to meet expected deadlines, or fail to comply with regulatory requirements, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for any other reasons, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize, any product candidate that we develop. As a result, our financial results and the commercial prospects for any product candidate that we develop would be harmed, our costs could increase, and our ability to generate revenue could be delayed. While we will have agreements governing their activities, our CROs will not be our employees, and we will not control whether or not they devote sufficient time and resources to our future clinical and preclinical programs. These CROs may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials, or other drug development activities which could harm our business. We face the risk of potential unauthorized disclosure or misappropriation of our intellectual property by CROs, which may reduce our trade secret protection and allow our potential competitors to access and exploit our proprietary technology.
If our relationship with any of these CROs terminates, we may not be able to enter into arrangements with alternative CROs or do so on commercially reasonable terms. Switching or adding additional CROs involves substantial cost and requires management time and focus. In addition, there is a natural transition period when a new CRO commences work. As a result, delays occur, which can negatively impact our ability to meet our desired clinical development timelines. While we intend to carefully manage our relationships with our CROs, there can be no assurance that we will not encounter
challenges or delays in the future or that these delays or challenges will not have a negative impact on our business, financial condition and prospects.
In addition, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services. Under certain circumstances, we may be required to report some of these relationships to the FDA. The FDA may conclude that a financial relationship between us and a principal investigator has created a conflict of interest or otherwise affected interpretation of the trial. The FDA may therefore question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of our marketing applications by the FDA and may ultimately lead to the denial of marketing approval of our product candidates.
Even if products using our technology are approved by the FDA or any other regulatory agency, we will be subject to additional ongoing regulatory obligations and oversight in the U.S. and other countries where we obtain approval.
For example, we may be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval, or requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor the safety and efficacy of the product candidate. In addition, if the FDA approves a product candidate, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion, and recordkeeping for the product will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration, and continued compliance with FDA cGMPs, good clinical practices (GCPs), and good laboratory practices, which are regulations and guidelines enforced by the FDA for all products in clinical development and for any clinical trials that we conduct post-approval. In addition, post-marketing requirements for our product candidates may include implementation of a Risk Evaluation and Mitigation Strategies (REMS) to ensure that the benefits of the product outweigh its risks. A REMS may include a Medication Guide, a patient package insert, a communication plan to healthcare professionals, and/or other elements to assure safe use of the product. Compliance with all these requirements, and any other requirements imposed upon us by U.S. or overseas regulators, could be costly to us, and failure to comply with these requirements could cause us to lose any marketing approval that we may have obtained, subject us to sanctions and jeopardize our ability to commercialize our product candidates.
Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with any third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
•refusals or delays in the approval of applications or supplements to approved applications;
•refusal of a regulatory authority to review pending market approval applications or supplements to approved applications;
•restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market or voluntary or mandatory product recalls or seizures;
•fines, warning letters, or holds on clinical trials;
•import or export restrictions;
•injunctions or the imposition of civil or criminal penalties;
•restrictions on product administration, requirements for additional clinical trials, or changes to product labeling, or REMS programs; or
•recommendations by regulatory authorities against entering into governmental contracts with us.
Even if we obtain regulatory approval for our product candidates using our technology in the United States, our ability to market a product would be limited to those uses that are approved for that product.
The FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, dissemination of off-label information, industry-sponsored scientific and educational activities, and promotional activities involving the Internet. Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved label. In the United States, we intend to seek approval for products for various types of cancer. If the FDA approves any drug application, our ability to market and promote a product would be limited to the indication tested for a specific disease, so even with FDA approval, products using our technology may only be promoted in this limited market. Physicians may prescribe legally available drugs for uses that are not described in the product’s labeling, and that differ from those tested by us and approved by the FDA. Such off-label uses are common
across medical specialties, including oncology. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, impose stringent restrictions on manufacturers’ communications regarding promotion of approved drug products for off-label use, and FDA approval may otherwise limit our sales practices and our ability to promote, sell, and distribute a product. Thus, we may only market products using our technology, if approved by the FDA, for its approved indication and we could be subject to enforcement action for off-label marketing.
Further, if there are any modifications to an approved product, including changes in indications, labeling, or manufacturing processes or facilities, we may be required to submit and obtain FDA approval of a new or supplemental NDA, which may require us to develop additional data or conduct additional preclinical studies and clinical trials. Failure to comply with these requirements can result in regulatory enforcement actions and adverse publicity.
If future clinical trials are unsuccessful, significantly delayed or not completed, we may not be able to market products for other indications or our technology.
If we do not obtain required approvals in other countries in which we aim to market our product candidates, we will not be able to export or sell the products in those markets, which will limit our sales opportunities.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not guarantee that we will be able to obtain or maintain regulatory approval in any other jurisdiction, while a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. For example, even if the FDA grants marketing approval of a product candidate, similar foreign regulatory authorities must also approve the manufacturing, marketing and promotion of the product candidate in those countries. Approval and licensure procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the United States, including additional preclinical studies or clinical trials as clinical trials conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions.
Our lack of experience conducting clinical trials outside the United States and Canada may negatively impact the approval process in foreign countries where we intend to seek approval for the products using our technology. We have not previously conducted multi-national clinical trials.
If we are unable to obtain and maintain required approval from one or more foreign jurisdictions where we would like to sell products using our technology, we will be unable to market products as intended, our international market opportunity will be limited, and our results of operations will be harmed.
If no product candidates using our technology are approved by the FDA or other regulatory body, third-party payors in the United States or anywhere will not reimburse the use of our product candidates. Even if approval is obtained, inadequate reimbursement may harm results of operations.
Following regulatory approval, we intend to seek reimbursement by third-party payors for the products created by our technology. There are no assurances that third-party payors in the United States or other countries will agree to cover the cost of products using our technology at all or at rates that are adequate to cover actual costs. Further, third-party payors may deny reimbursement if they determine that our product candidates are not used in accordance with established payor protocols regarding cost effective treatment methods or are used outside their approved indication or for forms of cancer not specifically approved by the FDA or other foreign regulatory bodies in the future. Without reimbursement, physicians, hospitals, and other healthcare providers may be less likely to prescribe our product candidates thereby harming our results of operations. Without adequate reimbursement, we may not be able to successfully commercialize systems.
Risks Related to Manufacturing, Commercialization, and Market Acceptance of Products made using our Technology
We intend to rely on third parties to produce clinical and commercial supplies of our product candidates.
We do not own or operate facilities for drug manufacturing, storage and distribution, or testing. We are dependent on third parties to manufacture the clinical supplies of our current and any future product candidates. The facilities used by our contract manufacturers to manufacture our product candidates must be approved by the FDA pursuant to inspections that will be conducted after we submit our NDA to the FDA. We do not control the manufacturing process of, and are completely dependent on, our contract manufacturing partners for compliance with the cGMP requirements, for manufacture of both active drug substance and finished drug product. If our contract manufacturers cannot successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the FDA or others, we
will not be able to secure and/or maintain regulatory approval for our product candidates. In addition, we have no control over the ability of our contract manufacturers to maintain adequate quality control, quality assurance and qualified personnel. If the FDA or a comparable foreign regulatory authority does not approve these facilities for the manufacture of our product candidates or if it withdraws any such approval in the future, we may need to find alternative manufacturing facilities, which would significantly impact our ability to develop, obtain regulatory approval for or market our product candidates, if approved. Any significant delay in the supply of a product candidate, or the raw material components thereof, for an ongoing clinical trial due to the need to replace a third-party manufacturer could considerably delay completion of our clinical trials, product testing and potential regulatory approval of our product candidates.
We also intend to rely on third-party manufacturers to supply us with sufficient quantities of our product candidates to be used, if approved, for commercialization. We do not yet have a commercial supply agreement for commercial quantities of drug substance or drug product. If we are not able to meet market demand for any approved product, it would negatively impact our ability to generate revenue, harm our reputation, and could have an adverse effect on our business and financial condition.
Further, our reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured product candidates ourselves, including:
•inability to meet our product specifications and quality requirements consistently;
•delay or inability to procure or expand sufficient manufacturing capacity;
•issues related to scale-up of manufacturing;
•costs and validation of new equipment and facilities required for scale-up;
•our third-party manufacturers may not be able to execute our manufacturing procedures and other logistical support requirements appropriately;
•our third-party manufacturers may fail to comply with cGMP requirements and other inspections by the FDA or other comparable regulatory authorities;
•our inability to negotiate manufacturing agreements with third parties under commercially reasonable terms, if at all;
•breach, termination or nonrenewal of manufacturing agreements with third parties in a manner or at a time that is costly or damaging to us;
•reliance on single sources for drug components;
•lack of qualified backup suppliers for those components that are currently purchased from a sole or single-source supplier;
•our third-party manufacturers may not devote sufficient resources to our product candidates;
•we may not own, or may have to share, the intellectual property rights to any improvements made by our third-party manufacturers in the manufacturing process for our product candidates;
•operations of our third-party manufacturers or suppliers could be disrupted by conditions unrelated to our business or operations, including the bankruptcy of the manufacturer or supplier; and
•carrier disruptions or increased costs that are beyond our control.
In addition, if we enter into a strategic collaboration with a third party for the commercialization of our current or any future product candidates, we will not be able to control the amount of time or resources that they devote to such efforts. If any strategic collaborator does not commit adequate resources to the marketing and distribution of our product candidates, it could limit our potential revenues.
Any of these events could lead to clinical trial delays or failure to obtain regulatory approval, or impact our ability to successfully commercialize our current or any future product candidates once approved. Some of these events could be the basis for FDA action, including injunction, request for recall, seizure, or total or partial suspension of production.
We purchase components for our product candidates from third parties, some of which may be sole-source suppliers.
Our product candidate is comprised of three key ingredients, the excipient (referred to as SHAO) and two active, commercially available pharmaceutical ingredients cisplatin and vinblastine sulphate. Currently each of the three ingredients and our product candidate are single sourced. While we are aware of other suppliers for the two active
ingredients, those suppliers have not been qualified as yet. We also have identified other producers of both the SHAO excipient and the product candidate. We manufacture SHAO using Curia in Albany, New York and INT230-6 at Curia in Glasgow, Scotland. We have only qualified Curia to produce SHAO and INT230-6 at this time. We control the manufacturing processes for SHAO and INT230-6, and we have all information on the production of the molecule and product candidate; however, it would take several months to qualify a new supplier or suppliers. We purchase the cisplatin from Veranova in West Deptford, New Jersey. Veranova is the developer of cisplatin and one of the world’s largest producer of cisplatin. We have only qualified Veranova. We purchase vinblastine sulphate from Minakem located in Mont-Saint-Guibert, Belgium. We have only qualified Minakem as a supplier of our vinblastine sulphate. It would take several months to quality new vendors for cisplatin and vinblastine sulfate.
We rely and expect to continue to rely completely on third parties to manufacture key components of our preclinical, clinical trial and commercial product candidate supplies. The development and commercialization of any of our product candidates could be stopped, delayed or made less profitable if those third parties fail to provide us with sufficient quantities of such product supplies or fail to do so at acceptable quality levels, including in accordance with applicable regulatory requirements or contractual obligations, and our operations could be harmed as a result. The components of our product candidates, including enhancers, drugs, and excipients, must be manufactured and assembled in accordance with approved manufacturing and predetermined performance specifications and must meet CGMP and quality systems requirements. Some states also have similar regulations. Many of the other components of our product candidates may be manufactured by sole-source suppliers that may have proprietary manufacturing processes. If we need to find a new source of supply, we may face long interruptions in obtaining necessary components for our product candidates, in obtaining FDA or foreign regulatory agency approval of these components and in establishing the manufacturing process, which could jeopardize our ability to supply products using our technology to the market.
We have not entered into long term manufacturing and supply agreements with any producers.
We intend to pursue agreements with contract manufacturers to produce the components and drug products that we will use in the future for the commercialization of products that make using of our technology, as well as for labeling and finishing services. We may not be able to enter into such arrangements on acceptable terms or at all. Components of our product candidates are currently manufactured for us in small quantities for use in our preclinical and clinical studies. We will require significantly greater quantities to commercialize any given product. We may not be able to find alternate sources of comparable components. If we are unable to obtain adequate supplies of components from our existing suppliers or need to switch to an alternate supplier and obtain FDA or other regulatory agency approval of that supplier, commercialization of our product candidates may be delayed. If we are unable to obtain sufficient compounds and labeling services on acceptable terms, or if we should encounter delays or difficulties in our relationships with our current and future suppliers or if our current and future suppliers of each component do not comply with applicable regulations for the manufacturing and production of drugs, our business, financial condition, and results of operations may be materially harmed.
If we cannot successfully purchase or produce the drugs used in the manufacture of our product candidates, our ability to develop and commercialize products using our technology would be impaired.
To manufacture the therapeutic agents on our own, we would first have to develop a manufacturing facility that complies with FDA requirements and regulations to produce each therapeutic agent we choose to manufacture. Developing these resources would be an expensive and lengthy process and would have a material adverse effect on our revenues and profitability. We have no manufacturing history and we may not be able to scale up or demonstrate manufacture of commercial quantities, in a cost-effective manner, or in compliance with the regulatory requirements applicable to such manufacturing. Additionally, we may have difficulty obtaining other components for the system from our third-party suppliers in a timely manner or at all which may adversely affect our ability to conduct timely clinical trials in the United States and elsewhere to obtain regulatory approval, and our ability to deliver our product candidates to purchasers.
Our current and future relationships with investigators, health care professionals, consultants, third-party payors, and customers will be subject to applicable healthcare regulatory laws, which could expose us to penalties.
Our business operations and current and future arrangements with investigators, healthcare professionals, consultants, third-party payors, patient support, charitable organizations and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations. These laws regulate the business or financial arrangements and relationships through which we conduct our operations, including how we research, market, sell, and distribute our product candidates for which we obtain marketing approval. Such laws include, among others: he federal Anti-Kickback Statute, the federal false claims laws, including the False Claims Act, the federal Health Insurance Portability and Accountability
Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their implementing regulations, the federal Physician Payments Sunshine Act, federal consumer protection and unfair competition laws and analogous state and foreign laws and regulations, such as state antikickback and false claims laws, which may apply to our business practices. For additional information regarding the regulatory regime under which we operate, see “Business — Government Regulation.”
Efforts to ensure that our current and future business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations, agency guidance or case law involving applicable healthcare laws. If our operations are found to be in violation of any of these or any other health regulatory laws that may apply to us, we may be subject to significant penalties, including the imposition of significant civil, criminal and administrative penalties, damages, monetary fines, disgorgement, individual imprisonment, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs or similar programs in other countries or jurisdictions, contractual damages, reputational harm, diminished profits and future earnings, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. Even the mere issuance of a subpoena or the fact of an investigation alone, regardless of the merit, may result in negative publicity, a drop in our share price and other harm to our business, financial condition and results of operations. Defending against any such actions can be costly, time-consuming and may require significant financial and personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired.
If we or any contract manufacturers and suppliers we engage fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our research and development activities involve the use of biological and hazardous materials and produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials, which could cause an interruption of our commercialization efforts, research and development efforts and business operations, environmental damage resulting in costly clean-up and liabilities under applicable laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. Although we believe that the safety procedures utilized by our third-party manufacturers for handling and disposing of these materials generally comply with the standards prescribed by these laws and regulations, we cannot guarantee that this is the case or eliminate the risk of accidental contamination or injury from these materials. In such an event, we may be held liable for any resulting damages and such liability could exceed our resources and state or federal or other applicable authorities may curtail our use of certain materials and/or interrupt our business operations. Furthermore, environmental laws and regulations are complex, change frequently and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our future compliance. In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials or other work-related injuries, this insurance may not provide adequate coverage against potential liabilities. We do not carry specific biological waste or hazardous waste insurance coverage, workers compensation or property and casualty and general liability insurance policies that include coverage for damages and fines arising from biological or hazardous waste exposure or contamination.
We have limited experience in marketing and commercializing products and, as a result, we may not be successful in commercializing products made using our technology.
If we are unable to find a development or marketing partner, we may have to directly and indirectly market our product candidates. To pursue a direct marketing strategy in any country may require the engagement of a contract sales organization to provide medical science liaisons to educate the medical oncologists, and we may need to utilize a direct sales force to sell our product candidates to interventional radiologists and hospitals. However, we have not previously sold, marketed, or distributed any products and have limited experience in building a sales and marketing organization and in entering and managing relationships with third-party distributors. To pursue such a potential strategy, we must acquire or
internally develop a sales, marketing, and distribution infrastructure and/or enter into strategic alliances to perform these services. The development of sales, marketing and distribution infrastructure is difficult and time consuming and would require substantial financial and other resources. If we cannot successfully partner the products for marketing or develop the infrastructure to market and commercialize the products ourselves, our ability to generate revenues may be harmed, and we may be required to enter strategic alliances to have such activities carried out on our behalf, which may not be on favorable terms.
Even if we are successful in commercializing products using our technology in the United States, we may not be successful in other foreign countries.
Each country requires a different commercialization strategy, so our U.S. strategy may not translate to other markets. Without a successful commercialization strategy tailored for each market, our efforts to promote and market the products in each of our target markets may fail in any or all those markets.
Our plan to use collaborative arrangements with third parties to help finance and to market and sell products using our technology may not be successful.
Our efforts may never result in the successful development or commercialization of products using our technology. The success of any development program will depend upon our ability to perform our obligations under any agreements as well as factors beyond our control, such as the commitment of our vendor collaborators and the timely performance of their obligations. The terms of any such collaboration may permit our collaborators to abandon the alliance at any time for any reason or prevent us from terminating arrangements with vendors or collaborators who do not perform in accordance with our expectations or our collaborators may breach their agreements with us. In addition, any third parties with which we collaborate may have significant control over important aspects of the development and commercialization of our product candidates, including research and development, market identification, marketing methods, pricing, composition of sales force, and promotional activities. We are not able to control or influence the amount and timing of resources that any vendor or collaborator may devote to our research and development programs or the commercialization, marketing, or distribution of our product candidates. We may not be able to prevent any collaborators from pursuing alternative technologies or products that could result in the development of products that compete with our technology or the withdrawal of their support for our product candidates. The failure of any such collaboration could have a material adverse effect on our business.
We will be dependent on healthcare professionals’ efforts to learn about our product candidates.
As a result, the products being developed may not gain significant market acceptance among physicians, hospitals, patients and healthcare payors until healthcare professionals are properly educated about the procedures involved in using the products. Market acceptance of our product candidates and technology will depend upon a variety of factors including:
•whether our future clinical trials demonstrate significantly improved patient outcomes;
•our ability to educate and train physicians to perform the image guided injection procedures and drive acceptance of the use of products;
•our ability to convince healthcare payors that use of the technology results in reduced treatment costs and improved outcomes for patients;
•whether our system replaces and/or complements treatment methods in which many hospitals have made a significant investment; and
•whether doctors and hospitals are willing to replace their existing technology with a new medical technology until the new technology’s value has been demonstrated.
We may need to establish clinical training and centers of excellence to educate and train physicians and healthcare payors, but the key opinion thought leadership required for initial market acceptance within the healthcare arena may take time to develop.
Without effort from key opinion healthcare professionals to become educated about our product candidates, and guide physicians, the market may not accept our approach and our efforts to commercialize our product candidates may be unsuccessful. Similar considerations apply in any other market where we receive approval. Successful commercialization of the methodology in many markets will depend on market acceptance by thought leading healthcare professionals.
Healthcare legislative reform measures may have a negative impact on our business and results of operations.
In the United States and some foreign jurisdictions, there have been, and likely will continue to be, a number of legislative and regulatory changes and proposed changes regarding the healthcare system directed at broadening the availability of healthcare, improving the quality of healthcare, and containing or lowering the cost of healthcare. For example, in March 2010, the United States Congress enacted the ACA, which, among other things, includes changes to the coverage and payment for products under government health care programs. And since its enactment, there have been numerous judicial, administrative, executive, and legislative challenges to certain aspects of the ACA.
Other legislative changes have been proposed and adopted in the United States since the Affordable Care Act was enacted. In August 2011, the Budget Control Act of 2011, among other things, included aggregate reductions of Medicare payments to providers of 2% per fiscal year, which went into effect in April 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2031 unless additional Congressional action is taken. The Coronavirus Aid, Relief, and Economic Security Act, or CARES Act, which was signed into law on March 27, 2020, designed to provide financial support and resources to individuals and businesses affected by the COVID-19 pandemic, suspended these reductions from May 1, 2020 through December 31, 2020, and extended the sequester by one year, through 2030. In addition, in January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. Additionally, on March 11, 2021, President Biden signed the American Rescue Plan Act of 2021 into law, which eliminates the statutory Medicaid drug rebate cap, currently set at 100% of a drug’s average manufacturer price, for single-source and innovator multiple-source drugs, beginning January 1, 2024. These laws may result in additional reductions in Medicare, Medicaid and other healthcare funding.
Moreover, payment methodologies may be subject to changes in healthcare legislation and regulatory initiatives. For example, CMS may develop new payment and delivery models, such as bundled payment models. In addition, recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their commercial products, which has resulted in several Congressional inquiries and proposed and enacted state and federal legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for pharmaceutical products. For example, on July 24, 2020 and September 13, 2020, the Trump administration announced several executive orders related to prescription drug pricing and importation. As a result, the FDA also released a final rule in September 2020, effective November 30, 2020, providing guidance for states to build and submit importation plans for drugs from Canada. Further, in November 2020, the U.S. Department of Health and Human Services, or HHS, finalized a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The implementation of the rule has been postponed by Congress and HHS to January 1, 2032.
The CMS also issued an interim final rule that would have established a Most Favored Nation, or MFN, Model for Medicare Part B drug payments. This regulation would have substantially changed the reimbursement landscape as it would have based Medicare Part B payment for 50 selected drugs on prices in foreign countries instead of average sales prices (ASP) and established a fixed add-on payment in place of the current 6 percent (4.3 percent after sequestration) of ASP. The MFN drug payment amount was expected to be lower than the current ASP-based limit because U.S. drug prices are generally the highest in the world. However, CMS issued a final rule on December 29, 2021 that rescinded the MFN Model interim final rule.
In December 2020, CMS issued a final rule implementing significant manufacturer price reporting changes under the Medicaid Drug Rebate Program, including regulations that affect manufacturer-sponsored patient assistance programs subject to pharmacy benefit manager accumulator programs and Best Price reporting related to certain value-based purchasing arrangements. On May 21, 2021, an industry group sued CMS, claiming that the change to the Best Price rule exceeds CMS’s statutory authority and is contrary to the Medicaid Rebate statute, and on May 17, 2022, the U.S. District Court for the District of Columbia vacated the Best Price rule.
In August 2022, President Biden signed the Inflation Reduction Act of 2022 (IRA) into law. This statute marks the most significant action by Congress with respect to the pharmaceutical industry since adoption of the ACA in 2010. Among other things, the IRA requires manufacturers of certain drugs to engage in price negotiations with Medicare (beginning in 2026), with prices that can be negotiated subject to a cap; imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation (beginning October 1, 2022); and replaces the Medicare Part D coverage gap discount program with a new discounting program (beginning in 2025). The IRA permits the Secretary of the Department of Health and Human Services (HHS) to implement many of these provisions through guidance, as opposed to regulation, for the initial years. On March 15, 2023, and June 30, 2023, HHS issued guidance
regarding implementation of the Medicare drug price negotiation program in initial price applicability year 2026. HHS stated it would provide additional information in the future related to implementation for initial price applicability years 2027 and beyond. Some provisions of the IRA may be subject to legal challenge. Several manufacturers and industry groups have challenged the drug price negotiation program for Medicare Parts B and D in federal court. These lawsuits are ongoing, and additional lawsuits may be filed in the future. It is unknown whether such litigation or other litigation, if brought, will be successful. For these and other reasons, it is currently unclear how the IRA will be effectuated, and while the impact of the IRA on the pharmaceutical industry cannot yet be fully determined, it is likely to be significant.
On May 23, 2023, CMS issued a proposed rule that would modify many Medicaid Drug Rebate Program requirements and implement a drug price verification survey. It is unclear whether the proposed rule will be finalized and whether the final rule will differ from the proposed rule. It is also unclear to what extent these regulations or any future legislation or regulations will have on our business, including our ability to generate revenue and achieve profitability.
Outside the United States, ensuring coverage and adequate payment for a product also involves challenges. Pricing of prescription pharmaceuticals is subject to government control in many countries. Pricing negotiations with government authorities can extend well beyond the receipt of regulatory approval for a product and may require a clinical trial that compares the cost-effectiveness of a product to other available therapies. The conduct of such a clinical trial could be expensive and result in delays in commercialization.
In the European Union, pricing and reimbursement schemes vary widely from country to country. Some countries provide that products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to currently available therapies or so-called health technology assessments, in order to obtain reimbursement or pricing approval. For example, the European Union provides options for its member states to restrict the range of products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. European Union member states may approve a specific price for a product or they may instead adopt a system of direct or indirect controls on our profitability for placing the product on the market. Other member states allow companies to fix their own prices for products, but monitor and control prescription volumes and issue guidance to physicians to limit prescriptions. Recently, many countries in the European Union have increased the amount of discounts required on pharmaceuticals and these efforts could continue as countries attempt to manage healthcare expenditures, especially in light of the severe fiscal and debt crises experienced by many countries in the European Union.
We expect that these and other healthcare reform measures that may be adopted in the future may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved drug, which could have an adverse effect on demand for our product candidates. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our product candidates. For additional information on healthcare reform, see “Business — Government Regulation — Healthcare reform.”
If we fail to comply with our reporting and payment obligations under the Medicaid Drug Rebate Program or other governmental pricing programs in the U.S., we could be subject to additional reimbursement requirements, penalties, sanctions and fines which could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
In U.S. markets, our ability to commercialize our products successfully, and to attract commercialization partners for our products, should we choose to do so, depends in significant part on the availability of adequate financial coverage and reimbursement from third-party payors, including, in the U.S., governmental payors such as the Medicare and Medicaid programs, managed care organizations, and private health insurers. We therefore plan to participate in, and have drug price reporting, payment, and other compliance obligations under, these programs.
We plan to participate in the Medicaid Drug Rebate Program (MDRP). Under the MDRP, we will be required to pay a rebate to each state Medicaid program for our covered outpatient drugs that are dispensed to Medicaid beneficiaries and paid for by a state Medicaid program as a condition of having our drugs eligible for coverage under Medicaid and Medicare Part B. Those rebates will be based on pricing data that will be reported by us on a monthly and quarterly basis to the Centers for Medicare & Medicaid Services (CMS). If we become aware that our MDRP submissions for a prior period were incorrect or have changed as a result of recalculation of the pricing data, we will be required to resubmit the corrected data for up to three years after those data originally were due. Such restatements and recalculations increase our costs for complying with the laws and regulations governing the MDRP and the 340B program discussed below. Pursuant to the IRA, certain figures we report under the MDRP will also be used to compute rebates under Medicare Part D triggered by price increases that outpace inflation. If we fail to provide information timely or are found to have knowingly submitted
false information to CMS, we may be subject to civil monetary penalties and other sanctions, including termination from the MDRP.
Federal law requires that any company that participates in the MDRP also participate in the Public Health Service Act’s 340B drug pricing discount program (340B program), in order for the manufacturer’s drugs to be eligible for coverage under Medicaid and Medicare Part B. The 340B program is administered by the Health Resources and Services Administration (HRSA) and requires us to agree to charge statutorily defined covered entities no more than the 340B “ceiling price” for our covered drugs when used in an outpatient setting. These 340B covered entities include a variety of community health clinics and other entities that receive health services grants from the Public Health Service, as well as certain small rural hospitals and hospitals that serve a disproportionate share of low-income patients. For four eligible hospital types, certain free-standing cancer hospitals, critical access hospitals, rural referral centers and sole community hospitals, drugs designated under section 526 of the Federal Food, Drug and Cosmetic Act as “orphan drugs” are exempt from the ceiling price requirements. The 340B ceiling price is calculated using a statutory formula, which is based on pricing data we report under the MDRP and the rebate amount for the covered outpatient drug as calculated under the MDRP. In general, products subject to Medicaid price reporting and rebate liability are also subject to the 340B ceiling price requirement. We must report 340B ceiling prices to HRSA on a quarterly basis, and HRSA publishes them to 340B covered entities and state Medicaid programs. HRSA has finalized regulations regarding the calculation of the 340B ceiling price and the imposition of civil monetary penalties on manufacturers that knowingly and intentionally overcharge covered entities for 340B eligible drugs. HRSA has also finalized an administrative dispute resolution process through which 340B covered entities may pursue claims against participating manufacturers for overcharges. A recent court decision in the District Court of South Carolina, Genesis Health Care, Inc. v. Becerra, found that HRSA’s definition of “patient” as applied to the 340B Program was too broad and may result in covered entities expanding the number of individuals considered eligible to receive drugs purchased through the 340B Program, resulting in higher volumes of drugs purchased at the discounted 340B ceiling price. In addition, legislation may be introduced that, if passed, would further expand the 340B program, such as adding further covered entities or requiring participating manufacturers to agree to provide 340B discounted pricing on drugs when used in an inpatient setting.
In order for products to be eligible for coverage under the Medicaid and Medicare Part B programs and to be purchased by certain federal agencies and grantees, we must also participate in the Department of Veterans Affairs Federal Supply Schedule (FSS), pricing program. As a participant, we must list our covered (innovator and authorized generic) drugs on an FSS contract and charge no more than Federal Ceiling Price (FCP), to the Department of Veterans Affairs, Department of Defense, Public Health Service, and Coast Guard when those agencies purchase from the FSS contract or a depot contract. FCP is calculated based on non-federal average manufacturer price data, which we are required to submit quarterly and annually. In addition, because our products are available in the retail and specialty pharmacy setting, we are required to provide rebates to the Department of Defense for prescriptions dispensed to Tricare beneficiaries from Tricare retail network pharmacies under the Tricare Retail Refund Program. If a manufacturer participating in the FSS program fails to provide timely information or is found to have knowingly submitted false information, the manufacturer may be subject to civil monetary penalties.
Individual states continue to consider and have enacted legislation to limit the growth of healthcare costs, including the cost of prescription drugs and combination products. A number of states have either implemented or are considering implementation of drug price transparency legislation that may prevent or limit our ability to take price increases at certain rates or frequencies. Requirements under such laws include advance notice of planned price increases, reporting price increase amounts and factors considered in taking such increases, wholesale acquisition cost information disclosure to prescribers, purchasers, and state agencies, and new product notice and reporting. Such legislation could limit the price or payment for certain drugs, and a number of states are authorized to impose civil monetary penalties or pursue other enforcement mechanisms against manufacturers for the untimely, inaccurate, or incomplete reporting of drug pricing information or for otherwise failing to comply with drug price transparency requirements. If we are found to have violated state law requirements, we may become subject to penalties or other enforcement mechanisms, which could have a material adverse effect on our business.
Pricing and rebate calculations vary among products and programs. The calculations are complex and will often be subject to interpretation by us, governmental or regulatory agencies and the courts. We may be liable for errors associated with our submission of pricing data. If we are found to have knowingly submitted false pricing data to the Medicaid program or the FSS pricing program, or fail to submit pricing data on a timely basis, we may be subject to significant civil monetary penalties. Such failure also could be grounds for CMS to terminate our Medicaid drug rebate agreement, which is the agreement under which we would participate in the MDRP. In the event that CMS terminates our rebate agreement, our products may no longer be eligible for coverage under Medicaid or Medicare Part B. There can be no assurance that our submissions will not be found to be incomplete or incorrect.
Efforts to ensure that our business arrangements with third parties, and our business generally, will comply with applicable healthcare laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations, agency guidance or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. In addition, the requirements and penalties described above may affect our ability to profitably sell any product for which we obtain marketing approval.
Rapid technological developments in treatment methods for cancer and competition with other forms of cancer treatments could affect our ability to achieve meaningful revenues or profit.
Competition in the cancer treatment industry is intense. Products made using our technology will compete with all forms of cancer treatments that are alternatives to the “gold standard” treatment of surgical resection. Many of our competitors have substantially greater resources and considerable experience in conducting clinical trials and obtaining regulatory approvals. If these competitors develop more effective, more affordable products, or if treatment methods achieve earlier product development, our revenues or profitability will be substantially reduced.
The loss of key personnel could adversely affect our business.
The loss of any of our key members could delay our ability to develop the technology, conduct preclinical research, conduct clinical research, obtain FDA approval, or introduce products using our technology commercially and, ultimately, our ability to generate revenues and profits. Competition for experienced personnel is intense. If we cannot retain our current personnel or attract additional experienced personnel, our ability to compete could be adversely affected.
We are dependent on the services of our Chief Executive Officer, Lewis H. Bender, for the future success of our business. The loss of the services of Mr. Bender could have an adverse effect on our business, financial condition and results of operations. If that should occur, until we find another person to act as our chief executive officer, our operations could be suspended. In that event it is possible you could lose your entire investment.
Risks Related to Patents, Trade Secrets, and Proprietary Rights
Our success depends in part on our ability to obtain patents, maintain trade secret protection, operate without infringing on the proprietary rights of third parties, and commercialize our technology prior to the expiration of our patent protection.
We have three U.S. patents and one pending U.S. patent application. We have 12 foreign patents, including one European patent, validated in 27 countries. We have four pending foreign patent applications. We have registered trademarks and know-how. While we have patents and filed patent applications covering composition of matter, use and methods, only 15 patents have issued. Due to the uncertainty of the patent prosecution process, there are no guarantees that our pending patent applications or any future applications will result in the issuance of a patent. Even if we are successful in obtaining more U.S. patents and new patents in other countries, there is no assurance that our patents will be upheld if later challenged or will provide significant protection or commercial advantage. For example, given the uncertain situation in Eastern Europe, we cannot assure that our Russian patent will not be lost, given that payments necessary to maintain the patent may be unavailable in future years without the risk of international sanctions. Because of the length of time and expense associated with bringing new medical drugs and devices to the market, the healthcare industry has traditionally placed considerable emphasis on patent and trade secret protection for significant new technologies. Other parties may challenge our patents, patent claims or patent applications licensed or issued to us or may design around technologies we have patented, licensed or developed.
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions such as patent term
adjustments and/or extensions, may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired, we may be open to competition from competitive products, including biosimilars. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
If we do not obtain patent term extension and data exclusivity for any product candidates we may develop, our business may be materially harmed.
Depending upon the timing, duration and specifics of any FDA marketing approval of any product candidates we may develop, one or more of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Action of 1984 Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent extension term of up to five years as compensation for patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent may be extended and only those claims covering the approved drug, a method for using it, or a method for manufacturing it may be extended. However, we may not be granted an extension because of, for example, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents, or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or term of any such extension is less than we request, our competitors may obtain approval of competing products following our patent expiration, and our business, financial condition, results of operations, and prospects could be materially harmed.
Companies in the medical drug/device industry may use intellectual property infringement litigation to gain a competitive advantage.
In the United States, patent applications filed in recent years are confidential for 18 months, while older applications are not publicly available until the patent issues. As a result, even after the products using our technology are introduced to the market, there is no guarantee that we will be able to avoid patent infringement claims, whether such claims are ultimately held to have merit. Litigation may be necessary to enforce any patents issued or assigned to us or to determine the scope and validity of third-party proprietary rights. Litigation could be costly and could divert our attention from our business. There are no guarantees that we will receive a favorable outcome in any such litigation. If a third-party claims that we infringed its patents, any of the following may occur:
•we may become liable for substantial damages for past infringement if a court decides that our product candidates infringe upon a competitor’s patent;
•a court may prohibit us from selling or licensing our product candidates without a license from the patent holder, which may not be available on commercially acceptable terms or at all, or which may require us to pay substantial royalties or grant cross-licenses to our patents; and
•we may have to redesign our product candidate so that it does not infringe upon others’ patent rights, which may not be possible or could require substantial funds or time.
If a third party violates our intellectual property rights, we may be unable to enforce our rights because of our limited resources.
Use of our limited funds to enforce or to defend our intellectual property rights or to defend against legal proceedings alleging infringement of third party proprietary rights may also affect our financial condition adversely. If others file patent applications with respect to inventions for which we already have applications pending, we may be forced to participate in interference proceedings declared by the U.S. Patent and Trademark Office to determine priority of invention, which could also be costly and could divert our attention from our business. Because of the extensive time required for development, testing and regulatory review of a potential product, it is possible that, before the any product can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantages of the patent. Not all our U.S. patent rights will have corresponding patent rights effective in Europe or other foreign jurisdictions.
Similar considerations will apply in any other country where we may prosecute patent applications, may be issued patents, or may decide not to pursue patent protection relating to our technology. The laws of foreign countries may not protect our intellectual property rights to the same extent as do laws of the United States.
We protect our trade secrets and proprietary knowledge in part through confidentiality agreements with employees, consultants, and other parties. However, certain consultants, advisors and third parties with whom we have business relationships, and to whom in some cases we have disclosed or will disclose trade secrets and other proprietary knowledge, may also provide services to other parties in the medical device industry, including companies, universities, and research organizations that are developing competing products.
In addition, some employees may eventually seek employment with, and become employed by, our competitors. We cannot be assured that consultants, employees, and other third parties with whom we have entered into confidentiality agreements will not breach the terms of such agreements by improperly using or disclosing our trade secrets or other proprietary knowledge or that we will have adequate remedies for any such breach.
Trade secret protection does not prevent independent discovery of the technology or proprietary information or use of the same.
Competitors may independently duplicate or exceed our technology in whole or in part. If we are not successful in maintaining the confidentiality of our technology, the loss of trade secret protection or know-how relating to our technology will significantly impair our ability to commercialize our product candidates, and our value and results of operations will be harmed. Similar considerations apply in any other foreign country where we receive approval. Since we do not yet have valid issued patents for the products using our technology in some countries, our ability to successfully commercialize our technology in those countries may be harmed.
Risks Related to Products Liability
We may be the subject of product liability claims or product recalls, and we may be unable to maintain insurance adequate to cover potential liabilities.
Our business exposes us or may in the future expose us to potential liability risks that may arise from the testing, manufacture, marketing, sale and use of products using our technology. In addition, because certain products using the new technology are intended for use in patients with cancer, there is an increased risk of death among the patients treated with our system which may increase the risk of product liability lawsuits. We may be subject to claims against us even if the injury is due to the actions of others. For example, if the medical personnel that use our product candidates on patients are not properly trained or are negligent in the use of our product candidates, the patient may be injured through the use of our product candidates, which may subject us to claims. Were such a claim asserted we would likely incur substantial legal and related expenses even if we prevail on the merits. Claims for damages, whether or not successful, could cause delays in clinical trials and result in the loss of physician endorsement, adverse publicity and/or limit our ability to market and sell the system, resulting in loss of revenue. In addition, it may be necessary for us to recall products that do not meet approved specifications, which would also result in adverse publicity, as well as resulting in costs connected to the recall and loss of revenue. A successful products liability claim, or product recall would have a material adverse effect on our business, financial condition and results of operations. We currently carry product liability and clinical trial insurance coverage, but it may be insufficient to cover one or more large claims.
Risks Related to Our Securities
We are an “emerging growth company” as defined in the JOBS Act and a “smaller reporting company” as defined in the Securities Exchange Act of 1934, as amended, or the Exchange Act, and will be able to avail ourselves of reduced disclosure requirements applicable to emerging growth companies and smaller reporting companies, which could make our securities less attractive to investors and adversely affect the market price of our securities.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. We will remain an emerging growth company until the earlier of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (ii) the last day of the fiscal year following the fifth anniversary of the date of the completion of our IPO; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission, which means the market value of our Common Stock that is held by non-
affiliates exceeds $700 million as of the prior June 30. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:
•not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404;
•not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements;
•providing only two years of audited financial statements in addition to any required unaudited interim financial statements and a correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure;
•reduced disclosure obligations regarding executive compensation; and
•exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. In this report, we have not included all of the executive compensation-related information that would be required if we were not an emerging growth company.
We may choose to take advantage of some, but not all, of the available exemptions. We have taken advantage of reduced reporting burdens in this report. In particular, we have provided only two years of audited financial statements and have not included all of the executive compensation information that would be required if we were not an emerging growth company. We cannot predict whether investors will find our securities less attractive if we rely on these exemptions. If some investors find our securities less attractive as a result, there may be a less active trading market for our securities and the price of our securities may be more volatile.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to use the extended transition period for new or revised accounting standards during the period in which we remain an emerging growth company; however, we may adopt certain new or revised accounting standards early.
We are also a “smaller reporting company” as defined in the Exchange Act. We may continue to be a smaller reporting company even after we no longer qualify as an emerging growth company. We may take advantage of certain of the scaled disclosures available to smaller reporting companies until the fiscal year following the determination that our voting and non-voting Common Stock held by non-affiliates is more than $250 million measured on the last business day of our second fiscal quarter, or our annual revenues are more than $100 million during the most recently completed fiscal year and our voting and non-voting Common Stock held by non-affiliates is more than $700 million measured on the last business day of our second fiscal quarter.
So long as we qualify as an “emerging growth company” or a “smaller reporting company,” we may elect not to provide you with certain information, including certain financial information and certain information regarding compensation of our executive officers, that we would otherwise have been required to provide in filings we make with the SEC, which may make it more difficult for investors and securities analysts to evaluate our company. Further, as mentioned above, so long as we qualify as an “emerging growth company” our independent registered public accounting firm will not be required to provide an attestation report on the effectiveness of our internal control over financial reporting, which may increase the risk that material weaknesses or significant deficiencies in our internal control over financial reporting go undetected. We cannot predict if investors will find our securities less attractive because we may rely on these exemptions. If some investors find our securities less attractive as a result, there may be a less active trading market for our securities, and the price of our securities may be more volatile and may decline.
Substantial influence will remain with our management and major stockholder, which could delay or prevent a change of control or cause us to take actions in conflict with the intent of our stockholders.
The existing holdings of our executive officers, directors, principal stockholders and their affiliates represent beneficial ownership, in the aggregate, of up to approximately 47.9% of our outstanding Common Stock. Our President and CEO beneficially owns approximately 16.1% of our outstanding Common Stock. These stockholders, if they act together, will be able to influence our management and affairs and the outcome of matters submitted to our stockholders for approval, including the election of directors and any merger, consolidation or sale of all or substantially all of our assets.
These stockholders may have interests with respect to their Common Stock that are different from other investors. The concentration of voting power among these stockholders may have an adverse effect on the price of our Common Stock.
The price of our Common Stock may be volatile and fluctuate substantially, which could result in substantial losses for purchasers of our Common Stock.
Our stock price is likely to be volatile. The stock market in general and the market for biotechnology companies in particular have experienced extreme volatility. Due to our history of losses as well as a variety of factors, many of which are outside of our control and may be difficult to predict, our quarterly and annual operating results may fluctuate significantly in the future. This variability and unpredictability could also result in our failing to meet the expectations of industry or financial analysts or investors for any period. If our revenue or operating results fall below the expectations of analysts or investors or below any forecasts we may provide to the market, or if the forecasts we provide to the market are below the expectations of analysts or investors, the price of our Common Stock could decline substantially.
Further, investors in our Common Stock may experience a decrease, which could be substantial, in the value of their stock for reasons unrelated to our operating performance or prospects, and could lose part or all of their investment. The price of our Common Stock could be subject to wide fluctuations in response to a number of factors, including those described elsewhere in this report and others such as:
•variations in our operating performance and the performance of our competitors;
•actual or anticipated fluctuations in our quarterly or annual operating results;
•publication of research reports by securities analysts about us or our competitors or our industry;
•announcements by us, our competitors or our vendors of significant contracts, acquisitions, joint marketing relationships, joint ventures or capital commitments;
•our failure or the failure of our competitors to meet analysts’ projections or guidance that we or our competitors may give to the market;
•additions and departures of key personnel;
•strategic decisions by us or our competitors, such as acquisitions, divestitures, spin-offs, joint ventures, strategic investments or changes in business strategy;
•the passage of legislation or other regulatory developments affecting us or our industry;
•speculation in the press or investment community;
•changes in accounting principles;
•terrorist acts, acts of war or periods of widespread civil unrest;
•natural disasters and other calamities; and
•changes in general market and economic conditions.
As a result of this volatility, you may not be able to sell your Common Stock at or above the your purchase price.
Sales of a substantial number of shares of our Common Stock by our existing stockholders in the public market could cause our stock price to fall.
As of March 2, 2024, we have outstanding a total of 13,709,377 shares of Common Stock. If our existing stockholders sell, or indicate an intention to sell, substantial amounts of our Common Stock in the public market, the trading price of our Common Stock could decline.
Our management will have broad discretion in using the cash and cash equivalents and investments and may not use these proceeds effectively, which could affect our results of operations and cause our stock price to decline.
We will have considerable discretion in the application of our cash and cash equivalents and investments. We intend to use our cash and cash equivalents and investments to fund discovery and clinical development efforts as well as to further expand our manufacturing platform and capabilities, to grow our infrastructure to support our pipeline, and to fund new and ongoing research activities, working capital and other general corporate purposes, which may include funding for the hiring of additional personnel, capital expenditures and the costs of operating as a public company. As a result, investors will be relying upon management’s judgment with only limited information about our specific intentions for the
use of the balance of the net proceeds of our cash and cash equivalents and investments. We may use our cash and cash equivalents and investments for purposes that do not yield a significant return or any return at all for our stockholders. In addition, pending their use, we may invest our cash and cash equivalents and investments in a manner that does not produce income or that loses value.
We do not anticipate paying dividends in the foreseeable future.
We do not anticipate paying dividends on our Common Stock in the foreseeable future. Therefore, in the absence of an acquisition transaction, the only way to realize a return on investment might be for investors to sell the stock, but it is unknown when, if ever, investors will be able to do so.
Provisions in our charter documents and Delaware law may deter takeover efforts that could be beneficial to stockholder value.
Our amended and restated certificate of incorporation and second amended and restated by-laws and Delaware law contain provisions that could make it harder for a third party to acquire us, even if doing so might be beneficial to our stockholders. These provisions include a classified board of directors and limitations on actions by our stockholders. In addition, our board of directors has the right to issue preferred stock without stockholder approval that could be used to dilute a potential hostile acquirer. Our certificate of incorporation also imposes some restrictions on mergers and other business combinations between us and any holder of 15.0% or more of our outstanding Common Stock. As a result, you may lose your ability to sell your stock for a price in excess of the prevailing market price due to these protective measures, and efforts by stockholders to change our direction or management may be unsuccessful. See the section entitled “Description of Securities” in this Annual Report.
Our amended and restated certificate of incorporation provides that the Court of Chancery of the State of Delaware will be the exclusive forum for certain disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our amended and restated certificate of incorporation provides that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware is the exclusive forum for the following types of actions or proceedings under Delaware statutory or common law:
•any derivative claim or cause of action brought on our behalf;
•any claim or cause of action for a breach of fiduciary duty owed by any of our current or former directors, officers or other employees to us or our stockholders;
•any claim or cause of action against us or any of our current or former directors, officers or other employees arising out of or pursuant to any provision of the Delaware General Corporation Law, our amended and restated certificate of incorporation or our second amended and restated bylaws (as each may be amended from time to time);
•any claim or cause of action seeking to interpret, apply, enforce or determine the validity of our amended and restated certificate of incorporation or our second amended and restated bylaws (as each may be amended from time to time, including any right, obligation or remedy thereunder);
•any claim or cause of action as to which the Delaware General Corporation Law confers jurisdiction to the Court of Chancery of the State of Delaware; and
•any claim or cause of action against us or any of our current or former directors, officers or other employees governed by the internal-affairs doctrine.
This provision would not apply to suits brought to enforce a duty or liability created by the Securities Act, the Exchange Act or any other claim for which the U.S. federal courts have exclusive jurisdiction. In addition, unless we consent in writing to the selection of an alternative forum, to the fullest extent permitted by law, the federal district courts of the United States of America shall be the exclusive forum for the resolution of any complaint asserting a cause or causes of action arising under the Securities Act, including all causes of action asserted against any defendant to such complaint.
For the avoidance of doubt, this provision is intended to benefit and may be enforced by us, our officers and directors, the underwriters to any offering giving rise to such complaint, and any other professional entity whose profession gives authority to a statement made by that person or entity and who has prepared or certified any part of the documents underlying the offering. However, these choice of forum provisions may limit a stockholder’s ability to bring a claim in a
judicial forum that it finds favorable for disputes with us or our directors, officers, or other employees. Further, these choice of forum provisions may increase the costs for a stockholder to bring such a claim and may discourage them from doing so.
While the Delaware courts have determined that such choice of forum provisions are facially valid, a stockholder may nevertheless seek to bring a claim in a venue other than those designated in the exclusive forum provisions, and there can be no assurance that such provisions will be enforced by a court in those other jurisdictions. If a court were to find the choice of forum provision contained in our amended and restated certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions. For example, the Court of Chancery of the State of Delaware recently determined that the exclusive forum provisions of federal district courts of the United States of America for resolving any complaint asserting a cause of action arising under the Securities Act is not enforceable. We note that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder.
Our board of directors could issue additional shares of Common Stock or a new class of preferred stock and dilute the equity positions of current stockholders without consent of the investors.
In the future, we expect to need additional funding, which we may obtain through the authorization and issuance of additional common or preferred equity securities. The authorization of additional shares of stock under our certificate of incorporation may be made without the affirmative vote of all the investors. Any issuance of additional shares of stock could dilute the equity position of our current stockholders. A future issuance of shares of preferred stock will result in the shares of our Common Stock being subject to certain preferential rights of such preferred stock, including a right to participate in the proceeds of any sale or liquidation of the Company ahead of the shares of Common Stock.
Our net operating loss carryforwards might not be able to be utilized in the future.
As of December 31, 2023, the Company had $32.0 million in both Federal and Connecticut net operating loss (“NOL”) carryforwards. The Internal Revenue Code (the “IRC”) contains limitations on the use of net operating loss carryforwards after the occurrence of substantial ownership changes as defined by IRC Section 382. Utilization of such operating loss carryforwards may be limited if such capital raises are determined to be a change in ownership under IRC Section 382. The Company has not completed an analysis under Section 382 of the Code.
THE SELECTED LIST OF RISK FACTORS ABOVE DOES NOT PURPORT TO BE A COMPLETE LIST OF ALL MATERIAL RISKS INHERENT WITH AN INVESTMENT IN OUR STOCK. WE URGE YOU TO CAREFULLY CONSIDER THESE RISKS AS WELL AS OTHERS COMMON TO EARLY STAGE VENTURES AND OTHER INVESTMENTS OF SIMILAR NATURE.
Item 1B. Unresolved Staff Comments
None.
Item 1C. Cybersecurity
Risk Management and Strategy
The Company is a late-stage clinical biotechnology company committed to applying scientific leadership in the field of localized cancer reduction leading to anti-cancer immune activation. Currently, management has not adopted a formal cybersecurity risk management program or process for assessing cybersecurity risk. Management assesses material risks from cybersecurity threats on an ongoing basis, including any potential unauthorized access to or occurrence on or conducted through the Company’s information systems that may result in adverse effects on the confidentiality, integrity, or availability of information systems or any information residing therein. To this end, the Company utilizes an outsourced information technology consultant to implement systems and procedures designed to reduce, respond to and monitor for cybersecurity threats and vulnerabilities. The outsourced information technology consultant conducts proactive patching and monitoring of all of our existing systems and has implemented systems and procedures to mitigate cybersecurity risks that the Company believes are appropriate for a company of our size, stage of growth and financial condition. In addition, the Company carries insurance with coverage for cyber events that it believes is suitable for a company of our size, stage of growth and financial condition.
As of the date of this Annual Report on Form 10-K, the Company is not aware of any cybersecurity threats, including as a result of any previous cybersecurity incidents, that have materially affected the Company, including the Company’s business strategy, results of operations or financial condition.
Governance
Management is responsible for the day-to-day management of the risks we face, while our Board of Directors as a whole has responsibility for the oversight of risk management, including as to material risks from cybersecurity threats. In its risk oversight role, the Company’s Board of Directors has the responsibility to satisfy itself that the risk management processes designed and implemented by management are appropriate and functioning as designed. The Board of Directors has delegated to the Audit Committee of the Board of Directors the responsibility for the oversight of information technology (including cybersecurity) risks. In general, the Company seeks to address cybersecurity risks through a cross-functional approach that is focused on preserving the confidentiality, integrity, and availability of the information that it collects and stores by identifying, preventing, and mitigating cybersecurity threats and effectively responding to cybersecurity incidents when they occur.
Item 2. Properties
In January 2017, the Company entered into a lease for approximately 2,500 square feet of office space in Westport, Connecticut, which was subsequently extended and increased to approximately 4,000 square feet. In June 2023, the Westport Lease was terminated.
In July 2023, the Company signed a 5.5 year lease for approximately 2,700 square feet of office space in Shelton, Connecticut. The initial monthly base rent payments are zero for the first six months, $2,910 for each of the next six months, and gradually increase to $3,275 per month for the last twelve months. The lease commencement date was September 1, 2023. The Company also pays a pro-rata share of common area maintenance, real estate taxes, and insurances which are treated as non-lease components and recorded as variable facilities costs on a monthly basis. The Company has a one-time option to cancel the Shelton Lease after 36 months if it provides written notice before the end of month 30.
Item 3. Legal Proceedings
None.
Item 4. Mine Safety Disclosures
Not applicable.
Part II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information and Holders
Our Common Stock is currently listed on the Nasdaq under the symbol “INTS.” On March 7, 2024, the closing price of our common stock, as reported by the Nasdaq was $5.58 per share and we had approximately 44 record holders of our common stock. The number of record holders was determined from the records of our transfer agent and does not include beneficial owners of common stock whose shares are held in the names of various security brokers, dealers, and registered clearing agencies. Continental Stock Transfer & Trust Company is the transfer agent and registrar for our common stock.
Dividend Policy
We have not paid any cash dividends on our Common Stock to date. We may retain future earnings, if any, for future operations, expansion and debt repayment and has no current plans to pay cash dividends for the foreseeable future. Any decision to declare and pay dividends in the future will be made at the discretion of the Board and will depend on, among other things, our results of operations, financial condition, cash requirements, contractual restrictions and other factors that the Board may deem relevant. In addition, our ability to pay dividends may be limited by covenants of any future outstanding indebtedness we or our subsidiaries incur. We do not anticipate declaring any cash dividends to holders of the Common Stock in the foreseeable future.
Unregistered Sales of Equity Securities and Use of Proceeds
On March 16, 2023, the Company entered into a convertible debt agreement with a holder for aggregate principal of $50,000. The outstanding principal balance together with the unpaid and accrued interest of the notes converted upon the completion of our IPO at a conversion price equal to 70% of our IPO price, for a total of 14,696 shares of common stock.
On March 30, 2023, the Company entered into a convertible debt agreement with a holder for aggregate principal of $155,000. The outstanding principal balance together with the unpaid and accrued interest of the notes converted upon the completion of our IPO at a conversion price equal to 70% of our IPO, for a total of 45,389 shares of common stock.
On April 1, 2023, the Company entered into a convertible debt agreement with its landlord for aggregate principal of $12,552. The outstanding principal balance together with the unpaid and accrued interest of the notes was converted upon the commencement of the Company’s IPO at a conversion price equal to 70% of its initial public offering price, for a total of 3,631 shares of common stock.
On May 11, 2023, the Company entered into a convertible debt agreement with a holder for aggregate principal of $25,000. The outstanding principal balance together with the unpaid and accrued interest of the notes was converted upon the commencement of the Company’s IPO at a conversion price equal to 70% of its initial public offering price, for a total of 7,228 shares of common stock.
In August 2023, the Company received aggregate proceeds of $50,000 upon the exercise of (i) 12,500 warrants to purchase shares of Common Stock at an exercise price of $2.00 by a consultant, and (ii) 12,500 options to purchase shares of Common Stock at an exercise price of $2.00 by a consultant.
In October 2023, the Company granted an aggregate of 116,000 warrants (the “Consultant Warrants”) to purchase shares of its Common Stock to three consultants of the Company in consideration of their services to the Company. The Consultant Warrants have an expiration date ten years from the grant date, and have an exercise price of $6.13. The vesting schedule for the Consultant Warrants are as follows: (i) two of the three consultants have 4,000 Consultant Warrants that vested immediately on the grant date and (ii) each of the three Consultants will have 36,000 of their warrants vest in equal monthly installments, beginning one month from the grant date.
The above securities were issued without registration under the Securities Act of 1933, as amended (the “Securities Act”), or any state securities laws in a transaction not involving a public offering and the Sellers represented they are an accredited investor. We relied on the exclusion from the registration requirements of the Securities Act of 1933 afforded by Section 4(a)(2).
On June 29, 2023, our Registration Statement on Form S-1, as amended (File No. 333-260565), was declared effective in connection with our IPO, pursuant to which we sold an aggregate of 3,900,000 shares of common stock to The
Benchmark Company, LLC, as representative of the underwriters (the “Representative”), at a public offering price of $5.00 per share for total gross proceeds of $19,500,000. On July 10, 2023, we sold an additional 585,000 shares of common stock to the Representative in connection with its exercise in full of its over-allotment option at a public offering price of $5.00 per share for additional gross proceeds of $2,925,000. The net proceeds from our IPO were used primarily to (i) initiate and conduct studies related to its therapeutic treatments, (ii) conduct clinical trials and operations, (iii) develop its product candidates, and (iv) fund its working capital and general corporate activities.
Issuer Purchases of Equity Securities
None.
Item 6. [Reserved]
Not applicable, reserved.
Item 7. Management’s Discussion And Analysis Of Financial Condition And Results Of Operations
The following discussion and analysis of our financial condition and results of operations should be read together with our financial statements and related notes appearing elsewhere in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K,including information with respect to our plans and strategy for our business and financing needs, includes forward-looking statements that involve risks and uncertainties and should be read together with the “Risk Factors” section of this Annual Report on Form 10-K for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of various factors, including those discussed below and elsewhere in this Annual Report and in other reports we file with the Securities and Exchange Commission, particularly those under “Risk Factors.”
Overview
Intensity Therapeutics, Inc. is a late-stage clinical biotechnology company passionately committed to applying scientific leadership in the field of localized cancer reduction leading to anti-cancer immune activation. Our new approach involves the direct injection into tumors of a unique product created from our DfuseRxSM discovery platform.
IT treatment, or treatment designed to contain a drug inside a tumor without spreading to the rest of the body, has been an objective of clinicians since discovery of chemotherapeutic agents. The challenge with IT treatment approaches is that a tumor’s lipophilic, high fat, dense and pressurized microenvironment is incompatible with and does not absorb water-based products. We believe that this drug delivery challenge limits the effectiveness of prior and current IT treatments, which involve injecting aqueous drugs into a tumor without sufficient consideration of the tumor environment (regardless of the drug’s mechanism or approach, i.e. the stimulation of an inflammatory response or efforts to attract immune cells into a hostile live tumor). Accordingly, there remains a continued unmet need for the development of direct IT therapies for solid tumors that provide high local killing efficacy coupled with nontoxic systemic anti-cancer effects. We believe we have created a product candidate with the necessary chemistry to overcome this local delivery challenge. Evidence shows the mechanism of tumor killing achieved by our drug candidate also leads to systemic immune activation and T-cell repertoire expansion in certain cancers.
Our platform creates patented anti-cancer product candidates comprising active anti-cancer agents and amphiphilic molecules. Amphiphilic molecules have two distinct components: one part is soluble in water and the other is soluble in fat or oils. When an amphiphilic compound is mixed with therapeutic agents, such as chemotherapies, the agents also become soluble in both fat and water. Our product candidates include novel formulations consisting of potent anti-cancer drugs mixed together with these amphiphilic agents.
Our lead product candidate, INT230-6, is primarily comprised of three components: (i) cisplatin, a proven anti-cancer cytotoxic agent, (ii) vinblastine sulfate, also a proven anti-cancer cytotoxic agent, and (iii) SHAO which enables the two cytotoxic agents to disperse through a tumor and diffuse into cancer cells following a direct intratumoral injection. These three components are mixed and combined into one vial at a fixed ratio. Cisplatin and vinblastine sulfate are both generic and available to purchase in bulk supply commercially. The FDA has approved both drugs as intravenous agents for several types of cancers. Cisplatin was first approved in 1978 for testicular cancer, and is also approved in ovarian and bladder cancer. The drug is also used widely in several other cancers including pancreatic and bile duct cancer. Vinblastine
sulfate was first approved in 1965 and is also approved in generalized Hodgkin’s disease, lymphocytic lymphoma, advanced carcinoma of the testis, and certain types of sarcoma. The drug is also used in breast and lung cancer.
In 2017, we initiated clinical study IT-01 using INT230-6 in the United States under an IND authorized by the FDA and in Canada under a CTA approved by Health Canada. Study IT-01 tested the safety and efficacy of INT230-6 in patients with refractory or metastatic cancers, and enrolled 110 patients in three arms: (i) INT230-6 used as a monotherapy, (ii) INT230-6 in combination with Merck’s Keytruda® (pembrolizumab), and (iii) INT230-6 in combination with BMS Yervoy® (ipilimumab). We completed enrollment of IT-01 in June 2022, locked the IT-01 database in February 2023 and finalized the clinical study report in September 2023. We delivered the combination-specific reports and other information to our partners in the fourth quarter of 2023.
In 2021, we initiated the INVINCIBLE 2 Study. The study enrolled 91 subjects and the database was locked in November 2023. The key endpoint was whether INT230-6 could reduce a patient’s cancer compared to no treatment (the current SOC) or a saline injection. Substantial reduction of cancer presurgically in aggressive forms of cancer has been shown to correlate with delaying disease recurrence. Other endpoints of the INVINCIBLE 2 Study were to understand the percentage of necrosis that can be achieved in tumors for a given dose, especially tumors larger than 2 centimeters in longest diameter, and whether either a local or whole body anti-cancer immune response could be induced. The INVINCIBLE 2 Study demonstrated a high order of necrosis in presurgical breast cancer tumors in the period from diagnosis to surgery, with some patients experiencing greater than 95% necrosis of the tumor. Data from the INVINCIBLE 2 Study demonstrated that INT230-6 had a favorable safety profile. An increase of certain types of immune cells (CD4+ and NK T-cells) in the tumor and blood was also shown. There was also an increase in the T-cells repertoire relative to control.
In mid-2024, we intend on initiating the INVINCIBLE-3 Study in certain metastatic soft tissue sarcoma subtypes. We plan to enroll 333 patients with an endpoint of overall survival.
Also in mid-2024, we intend on initiating IT-04. The endpoint for the Phase 2 portion of the IT-04 study is the change in the pathological complete response rate for the combination compared to the SOC alone. We expect to initiate the Phase 2 portion of the IT-04 study in mid-2024, which will provide data to size the Phase 3 portion of the IT-04 study.
We have also successfully developed Phase 3 quality analytical methods for the three INT230-6 components and successfully manufactured a large-scale batch of INT230-6. In a meeting with the FDA in the fourth quarter of 2023, we agreed on a CMC plan for Phase 3 and product registration for our three key ingredients and INT230-6. If we successfully execute the agreed upon plan, the CMC portion of an NDA should be acceptable to the FDA for product approval and registration (subject to final NDA review).
Since our inception in 2012, our operations have included business planning, hiring personnel, raising capital, building our intellectual property portfolio, and performing both research and development on our product candidates. We have incurred net losses since inception and expect to incur net losses in the future as we continue our research and development activities. To date, we have funded our operations primarily through approximately $54.5 million in cash received from the net proceeds of sales of our common stock, preferred stock and convertible notes. As of December 31, 2023, we had approximately $8.6 million of cash and cash equivalents plus approximately $6.2 million in investments in U.S. Treasury bills. Since our inception, we have incurred significant operating losses. We incurred net losses of $10.5 million and $7.6 million for the years ended December 31, 2023 and 2022, respectively. As of December 31, 2023 and 2022 we had an accumulated deficit of approximately $50.5 million and $38.7 million, respectively. We expect to incur significant expenses and operating losses for the next several years. See “Funding Requirements” below.
We expect our expenses to increase as we continue to:
•Initiate Phase 3 programs in sarcoma and/or breast cancer;
•Incur manufacturing costs for additional GMP batches of our product candidates and enhancer molecules;
•Seek regulatory approvals for any of our product candidates that successfully complete clinical trials;
•Hire additional personnel;
•Expand our operational, financial, and management systems;
•Invest in measures to protect our existing and new intellectual property; and
•Establish a sales, marketing, medical affairs, and distribution infrastructure to commercialize any product candidates for which we may obtain marketing approval and intend to commercialize.
Our ability to ultimately generate revenue to achieve profitability will depend heavily on the development, approval, and subsequent commercialization of our product candidates. If we fail to become profitable or are unable to sustain
profitability on a continuing basis, then we may be unable to continue our operations at planned levels and be forced to reduce or terminate our operations.
As a result, we will need substantial additional funding to support our continuing operations and pursue our growth strategy. Until such time as we can generate significant revenue from product sales, if ever, we expect to finance our operations through the sale of equity, debt financing, or other capital sources, which may include collaborations with other companies or other strategic transactions. We may not be able to raise additional funds or enter into such other agreements or arrangements when needed on favorable terms, or at all. If we fail to raise capital or enter into such agreements as and when needed, we would have to significantly delay, reduce, or eliminate the development and commercialization of one or more of our product candidates.
Components of Results of Operations
Revenue
To date, we have not generated any revenue from product sales and we do not expect any revenue from the sale of product in the foreseeable future. We have not generated any revenue from licensing of our technology or product candidates yet either. If our development efforts for any of our product candidates are successful and result in regulatory approval, then we may generate revenue in the future from product sales or licensing. We cannot predict if, when, or to what extent we will generate revenue from the commercialization, licensing or sale of any of our product candidates. We may never succeed in obtaining regulatory approval for any of our product candidates.
Research and Development Expenses
Salaries and Benefits
Salaries and benefits include employee-related expenses such as salaries and related benefits for employees engaged in research and development functions.
Clinical Trial Expenses
Clinical trial expenses includes payments to third parties in connection with the clinical development of our product candidates, including CROs, and costs due to clinical trials for patient care.
Contract Manufacturing
Contract manufacturing includes:
•Manufacturing of products for use in our preclinical studies and clinical trials, including payments to CMOs;
•Manufacture of new enhancer compounds;
•Manufacture and labelling of GMP product candidate;
•Product candidate stability testing of GMP batches; and
•Other costs such as shipping, storage, and analytical testing.
Consulting
Scientific consulting costs related to non-employees involved in research, including statistical analysis, clinical trial operations, development of product manufacturing techniques, and internet research related to oncology and chemistry issues that may impact our preclinical or clinical research.
Stock-Based Compensation
Stock-based compensation is the expense related to stock options granted to employees and warrants granted to independent consultants engaged in research and development functions.
General and Administrative Expenses
Salaries and Benefits
Salaries and benefits include employee-related expenses such as salaries and related benefits for employees engaged in fund raising, management, and corporate administration functions.
Legal Fees
Legal fees include to expenses for corporate, patent and trademark fees with outside law firms.
Accounting Fees
Accounting fees primarily include the cost of our independent auditors for our annual audit, quarterly reviews, and services related Securities and Exchange Commission filings, along with costs for income tax returns preparation, and the cost of maintaining our accounting system.
Consulting
Consulting are services provided by non-employees for general and administrative tasks. This includes human resources, finance, investor relations, board compensation, and internet support.
Insurance
Insurance includes directors and officers’ insurance, workers compensation insurance, product liability insurance, business insurance, employee and cyber liability insurance.
Other
Other general and administrative costs include facility expenses, office supplies, computer related costs, public relations costs, recruiting costs and conferences.
Stock-Based Compensation
Stock-based compensation is the expense related to stock options granted to our employees and board members and warrants granted to our independent consultants who work in the general and administrative aspects.
Other income and expenses
We earned interest income on our cash balances and investments in U.S. treasury bills.
We incurred interest expense on our convertible notes through June 29, 2023. Accrued interest was converted into common stock upon commencement of our IPO.
We accumulated federal research and development tax credits in prior tax years that are recoverable through a refund of Social Security taxes paid in current fiscal periods.
Results of Operations
The following tables summarize our results of operations for the years ended December 31, 2023 and 2022 (in thousands):
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| 2023 | | 2022 | |
| Operating expenses: | | | | | |
| Research and development | $ | 4,786 | | | $ | 5,132 | | | $ | (346) | |
| General and administrative | 3,533 | | | 2,418 | | | 1,115 | |
| Total operating expenses | 8,319 | | | 7,550 | | | 769 | |
| Loss from operations | (8,319) | | | (7,550) | | | (769) | |
| Interest income | 324 | | | 2 | | | 322 | |
| Interest expense | (305) | | | (82) | | | (223) | |
| Loss on debt extinguishment | (2,262) | | | - | | | (2,262) | |
| Other | 24 | | | 48 | | | (24) | |
| Net loss | $ | (10,538) | | | $ | (7,582) | | | $ | (2,956) | |
| Preferred stock deemed dividend | (1,324) | | | - | | | (1,324) | |
| Net loss attributable to common stockholders | $ | (11,862) | | | $ | (7,582) | | | $ | (4,280) | |
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| 2023 | | 2022 | |
| Research and development expenses: | | | | | |
| Salaries and benefits related costs | $ | 896 | | | $ | 1,180 | | | $ | (284) | |
| Clinical trial expenses | 1,987 | | | 2,838 | | | (851) | |
| Contract manufacturing | 922 | | | 98 | | | 824 | |
| Consulting | 267 | | | 213 | | | 54 | |
| Stock-based compensation | 714 | | | 803 | | | (89) | |
| $ | 4,786 | | | $ | 5,132 | | | $ | (346) | |
| | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | Change |
| 2023 | | 2022 | |
| General and administrative expenses: | | | | | |
| Salaries and benefits related costs | $ | 559 | | | $ | 340 | | | $ | 219 | |
| Legal fees | 424 | | | 623 | | | (199) | |
| Accounting fees | 427 | | | 347 | | | 80 | |
| Consulting | 407 | | | 326 | | | 81 | |
| Insurance | 608 | | | 75 | | | 533 | |
| Other | 424 | | | 342 | | | 82 | |
| Stock-based compensation | 684 | | | 365 | | | 319 | |
| $ | 3,533 | | | $ | 2,418 | | | $ | 1,115 | |
Year Ended December 31, 2023 Compared to Year Ended December 31, 2022
Research and development expenses decreased $0.3 million or 7%, and were primarily due to the following:
•Salaries and benefits related costs decreased $0.3 million due to four research employees leaving the Company in 2022 and 2023, which was partially offset by two new hires in late 2023 and bonus increases.
•Clinical trial expenses in our IT-01 study decreased $1.4 million due to the completion of enrollment in this study in mid-2022. This decrease was partially offset by $0.6 million in higher expenses for preliminary work related to our Phase 3 sarcoma study (IT-03).
•Contract manufacturing increased by $0.8 million due to costs for a new manufacturing batch of INT230-6 in 2023.
General and administrative expenses increased $1.1 million or 46%, and were primarily due to the following:
•Salaries and benefits related costs increased by $0.2 million due to salary and bonus increases and the hiring of a new chief financial officer in the fourth quarter of 2023, along with $0.3 million in higher stock-based compensation expense.
•Insurance increased by $0.5 million due to the additional directors and officers insurance as a publicly held company.
•Higher accounting fees, consulting and other expenses were partially offset by lower legal fees, as we completed our IPO in mid-2023 and transitioned into a publicly traded company.
Interest income increased $0.3 million due to interest earned on higher cash and investment balances from our IPO in June 2023. Interest expense decreased by $0.2 million due to convertible notes converting to common stock at the time of our IPO. In addition, we also recognized a $2.3 million loss on debt conversion at the time of the IPO.
At the time of our IPO, a preferred stock deemed dividend of $1.3 million was recognized, representing the value that was transferred to the Series B and C preferred stockholders upon triggering of anti-dilution provisions.
Liquidity and Capital Resources
Our financial statements have been prepared assuming we will continue as a going concern. We have incurred losses from operations and negative cash flows that raise substantial doubt about our ability to continue as a going concern.
Since our inception, we have not generated any revenue from product sales and have incurred significant operating losses. We expect to continue to incur significant expenses and operating losses for the foreseeable future as we advance the clinical development of our product candidates. We expect that our research and development and general and administrative costs will continue to increase significantly, including in connection with conducting clinical trials for our product candidates, developing our manufacturing capabilities and building and qualifying our manufacturing facility to support clinical trials and commercialization and providing general and administrative support for our operations, including the cost associated with operating as a public company. As a result, we will need additional capital to fund our operations, which we may obtain from additional equity or debt financings, collaborations, licensing arrangements or other sources.
We have financed our operations primarily through an initial investment from our founder, the issuance and sale of convertible debt notes, private equity financings, and the IPO, after which shares of our common stock began trading on Nasdaq under the symbol “INTS” on June 30, 2023. As of December 31, 2023, our cash, cash equivalents and investments were approximately $14.8 million. Based on our balances in cash, cash equivalents, and investments, we project to have sufficient cash to fund our current operating plan through the end of the first quarter of 2025.
The following table summarizes the net cash provided by (used in) operating activities and financing activities for the periods indicated (in thousands):
| | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 |
| Net cash used in operating activities | $ | (7,205) | | | $ | (5,477) | |
| Net cash used in investing activities | (6,023) | | | — | |
| Net cash provided by financing activities | 20,472 | | | 2,250 | |
| Net increase (decrease) in cash and cash equivalents | $ | 7,244 | | | $ | (3,227) | |
Operating Activities
Our cash used in operating activities for the year ended December 31, 2023 was $7.2 million, comprising of (i) our net loss of $10.5 million, as adjusted for $4.0 million in non-cash expenses (including $2.3 million for the conversion of convertible notes into shares of common stock, and $1.4 million for non-cash stock based compensation), and (ii) net changes in operating assets and liabilities of $0.6 million.
Our cash used in operating activities for the year ended December 31, 2022 was $5.5 million, comprising of (i) our net loss of $7.6 million, as adjusted for $1.3 million in non-cash expenses (including non-cash stock based compensation of $1.2 million), and (ii) net changes in operating assets and liabilities of $0.8 million.
Investing Activities
Our cash used in investing activities during the year ended December 31, 2023 totaled approximately $6.0 million and was primarily due to net purchases of marketable debt securities (net of redemptions of marketable debt securities).
There was no cash provided by or used in investing activities for the year ended December 31, 2022.
Financing Activities
Our cash provided by financing activities during the year ended December 31, 2023 was $20.5 million, primarily comprising of net proceeds of $20.2 million from our IPO in 2023, and $0.2 million from the sale of convertible notes prior to the IPO.
Our cash provided by financing activities during the year ended December 31, 2022 was $2.3 million from the issuance of convertible notes.
Off-Balance Sheet Arrangements
We did not have any off-balance sheet arrangements as of December 31, 2023.
Seasonality
Our business experiences limited seasonality.
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States, or U.S. GAAP. The preparation of our financial statements and related disclosures requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, costs and expenses, and the disclosure of contingent assets and liabilities in our financial statements. These items are monitored and analyzed by us for changes in facts and circumstances, and material changes in these estimates could occur in the future. We base our estimates on historical experience, known trends and events, and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We evaluate our estimates and assumptions on an ongoing basis. Our actual results may materially differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in the notes to our financial statements included elsewhere in this Annual Report, we believe that the following accounting policies are those most significant to the judgments and estimates used in the preparation of our financial statements.
Accrued Research and Development Expenses
Research and development costs are expensed as incurred. We record the estimated CRO, CMO, and patient care costs as services are provided but not yet invoiced and include these costs in the accrued expenses in the balance sheet and within research and development expense in the statement or operations.
Equity-Based Compensation
We recognize compensation costs related to stock option grants to employees and board members and warrant grants to nonemployees based on the estimated fair value of the awards on the date of grant. We estimate the grant date fair value and the resulting stock-based compensation expense using the Black-Scholes option-pricing model. The grant date fair value of the stock-based awards is recognized on a straight-line basis over the requisite service periods, which are generally the vesting period of the respective awards. Forfeitures are accounted for as they occur.
We historically have been a private company and lack company-specific historical and implied volatility information for our shares. Therefore, we estimate our expected share price volatility based on the historical volatility of publicly traded peer companies and expect to continue to do so until such time as we have adequate historical data regarding the volatility of our own traded share price.
JOBS Act Accounting Election
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Subject to certain conditions set forth in the JOBS Act, if, as an “emerging growth company”, we choose to rely on such exemptions we may not be required to, among other things, (i) provide an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404, (ii) provide all of the compensation disclosure that may be required of non-emerging growth public companies under the Dodd-Frank Wall Street Reform and Consumer Protection Act, (iii) comply with any requirement that may be adopted by the PCAOB regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis), and (iv) disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of the CEO’s compensation to median employee compensation. These exemptions will apply for a period of five years following the completion of our IPO or until we are no longer an “emerging growth company,” whichever is earlier.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Not applicable.
Item 8. Financial Statements and Supplementary Data
The information required by this Item is set forth beginning on page F-1 of this report and is incorporated herein by reference.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) that are designed to ensure that the information required to be disclosed by us in the reports filed or submitted by us under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and such information is accumulated and communicated to management, including the Chief Executive Officer, Chief Financial Officer, and Principal Accounting Officer, to allow timely decisions regarding required disclosure. Disclosure controls and procedures, no matter how well designed and operated, can provide only reasonable assurances of achieving the desired controls.
As of December 31, 2023, we carried out an evaluation over the effectiveness of the design and operation of our disclosure controls and procedures defined above. Based upon that evaluation, we have concluded that, as of December 31, 2023, our disclosure controls and procedures were not effective as a result of the material weaknesses identified in internal controls due to (i) a lack of segregation of duties due to limited administrative staff, (ii) limited reconciliation and review procedures over clinical contract accruals as we have rapidly expanded into new, late-stage clinical studies, and (iii) information technology matters regarding user access that aggregate to a material weakness.
Material Weakness and Remediation Plans
In response to the above identified weakness, we are taking the following remediation measures:
•We are reassessing our accounting procedures and, as part of the financial reporting process, plan to implement the use of supplementary checks and additional reviews and evaluations of transactions to improve the accuracy and reliability of our financial information.
•We are adding appropriate resources to ensure that such procedures are implemented and adequate reviews are performed.
•In December 2023, we hired a new Chief Financial Officer with extensive public-company reporting and technical accounting experience to provides additional financial reporting oversight and review.
•We have engaged additional technical accounting consultants to provide additional resources for the preparation and review of our quarterly close procedures.
•We will evaluate new accounting software systems to improve system controls, and have already implemented a new financial reporting and filing software platform to leverage system-controls and streamline quarterly SEC filings controls.
Our Chief Executive Officer, Chief Financial Officer, and Principal Accounting Officer will be active participants in these ongoing remediation processes and such processes will be subject to audit committee oversight. We believe these steps will improve the effectiveness of our internal controls. While we plan to take the above steps to remediate these weaknesses, we cannot assure you that we will be able to fully remediate them, which could impair our ability to accurately and timely meet our public company reporting requirements.
Limitations on the Effectiveness of Controls
Our management recognizes that any set of controls and procedures, no matter how well-designed and operated, can provide only reasonable, not absolute, assurance of achieving the desired control objectives. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, with us have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by management override of controls. For these reasons, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management’s Annual Report on Internal Control over Financial Reporting
This annual report does not include a report of management's assessment regarding internal control over financial reporting or an attestation report of the company's registered public accounting firm due to a transition period established by rules of the Securities and Exchange Commission for newly public companies.
Changes in Internal Control over Financial Reporting
Other than the remediations identified above, there have been no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the quarter ended December 31, 2023 covered by this report that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
None
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The following table sets forth the name, age (as of March 14, 2024) and position of individuals who currently serve as our directors and executive officers. The following also includes certain information regarding our directors’ and officers’ individual experience, qualifications, attributes and skills, and brief statements of those aspects of our directors’ backgrounds that led us to conclude that they should serve as directors.
| | | | | | | | | | | | | | |
Name | | Age | | Position |
| Lewis H. Bender | | 65 | | President, Chief Executive Officer and Chairman of the Board |
| Joseph Talamo | | 55 | | Chief Financial Officer |
| John Wesolowski | | 64 | | Principal Accounting Officer and Controller |
| James M. Ahlers | | 59 | | Executive Vice President of Corporate Finance |
| Dr. Emer Leahy | | 58 | | Director |
| Dr. Mark A. Goldberg | | 64 | | Director |
| Mr. Daniel Donovan | | 59 | | Director |
Executive Officers
Lewis H. Bender is our founder and has served as our President and Chief Executive Officer since April 2012. Prior to our founding, Mr. Bender was the CEO of publicly traded (AMEX & OTC) Interleukin Genetics, Inc. from 2008 until 2012. Interleukin was a personalized medicine company. Mr. Bender was successful in raising capital for us via a direct placement with institutional investors and partnered with the insurance industry for development of an IG product. Prior to joining Interleukin Genetics, Mr. Bender held numerous positions at Emisphere Technologies, Inc. at the time a publicly traded (Nasdaq) drug delivery company specializing in the development of oral delivery of poorly absorbed molecules. While at Emisphere from 1993 to December 2007, Mr. Bender held positions including Interim President & CEO, Chief Technology Officer, Senior Vice President of Business Development, and Vice President of Manufacturing and Process Development. Mr. Bender has over 26 years of biotech and pharmaceutical executive management experience. He has led development teams taking products from discovery to Phase 3 for compounds using novel drug delivery techniques. Mr. Bender has a both a BS and MS in Chemical Engineering from The Massachusetts Institute of Technology (MIT), an MBA from the University of Pennsylvania’s Wharton School, and an MA in International Studies also from the University of Pennsylvania. He is fluent in French and German. We believe that Mr. Bender’s immense experience in the biomedical and pharmaceutical industries, including at several publicly traded companies, qualifies him to serve on our Board.
Joseph Talamo has served as our Chief Financial Officer since December 2023. Prior to joining the Company, from August 2020 until November 2023, Mr. Talamo served as Senior Vice President and Chief Financial Officer of HiberCell, Inc., a clinical-stage biotechnology company developing therapeutics to address cancer relapse and metastasis. From June 2011 until July 2020, Mr. Talamo was employed by Lisata Therapeutics, Inc. (formerly known as Caladrius Biosciences, Inc.) where he served in various roles, including Corporate Controller and Chief Accounting Officer, and then later as the Company’s Senior Vice President and Chief Financial Officer. Mr. Talamo received a B.B.A. in Accounting from Hofstra University, and an M.B.A. in Finance from Hofstra University. Mr. Talamo is a Certified Public Accountant in the State of New York.
John Wesolowski has served as our Principal Accounting Officer and Controller since March 2017. In addition, Mr. Wesolowski served as our Interim Chief Financial Officer from June 2023 until December 2023. Prior to joining Intensity Therapeutics, from 1998 to 2016 Mr. Wesolowski was Director of Costing in the Yale University Controller’s office. In that role Mr. Wesolowski conducted financial reporting, property tax management, was responsible for calculations of overhead and benefit rates, and was involved in numerous special projects related to accounting process and controls. Also, at Yale, he was involved in financial reporting and the accounting matters related to clinical trials and other organized research. Prior to joining Yale Mr. Wesolowski was the Vice President and Controller for Automatic Fastener Corporation in Branford, CT from 1988 to 1998. In this role, Mr. Wesolowski oversaw all accounting, purchasing and human resource functions. John also has 5 years of experience in public accounting and auditing from working at KMG Main Hurdman, now KPMG. Mr. Wesolowski received a Bachelor of Science in Finance from The Pennsylvania State University (Penn State at University Park) and an MBA from the University of Connecticut in Management Science. He is a Certified Public Account since 1983.
James M. Ahlers has served as our Executive Vice President of Corporate Finance since June 2023 through a consulting agreement with Mr. Ahlers’ employer, Danforth Advisors LLC, a company that provides strategic and operational finance and accounting services to life science companies. Previously, Mr. Ahlers served as our Chief Financial
Officer from January 2022 until June 2023 through the consulting agreement. From February 2002 to November 2019, Mr. Ahlers served as Chief Financial Officer of Intarcia Therapeutics, Inc. Mr. Ahlers is an accomplished finance leader with 25 years of experience building life science businesses. During his career, he has managed capital raising transactions, including initial public offerings, that have raised in excess of $2 billion. In addition, he has developed and implemented international operations and global tax strategies. Mr. Ahlers holds a B.S. in accounting from the University of San Francisco.
Non-Employee Directors
Dr. Emer Leahy has served on our board of directors since June 2016. Dr. Leahy received her Ph.D. in Neuropharmacology from University College Dublin, Ireland, and her MBA from Columbia University. Since 2000, she has served as CEO of PsychoGenics Inc., a profitable preclinical CNS service company. She is also CEO of PGI Drug Discovery LLC, a company engaged in psychiatric drug discovery with five partnered clinical programs including one in Phase 3. Further, she holds an Adjunct Associate Professor of Neuroscience position at Mount Sinai School of Medicine. Dr. Leahy has more than 30 years of experience in drug discovery, clinical development and business development for pharmaceutical and biotechnology companies, including extensive knowledge of technology assessment, licensing, mergers and acquisitions, and strategic planning. Dr. Leahy served on the Emerging Companies Section Governing Board for the Board of Directors of the Biotechnology Industry Organization (BIO), the Business Review Board for the Alzheimer’s Drug Discovery Foundation, and the Scientific Advisory Board of the International Rett Syndrome Foundation. She also currently serves on the Board of Directors of PsychoGenics Inc., Bright Minds Biosciences, and Pasithea Therapeutics. Dr. Leahy also serves on the Board of Trustees of BIONJ, and effective February 2024, began serving in the role of Chair. We believe that Dr. Leahy’s extensive experience in the biopharmaceutical industry, including as a CEO of several companies, allows her to make valuable contributions to the Board.
Dr. Mark A. Goldberg has served as a member of our board of directors since May 2018. Since 2018, Dr. Goldberg has served as Chairman and CEO of Allucent, a global mid-sized clinical research organization. Dr. Goldberg has also served as the Executive Chairman of Thread, a decentralized research and electronic clinical outcome assessment provider, since 2019. Previously, Dr. Goldberg has served as President and COO of PAREXEL International, one of the world’s largest global biopharmaceutical service providers, with consolidated revenue of approximately $2.4 billion in 2017, over 18,000 employees, and 86 locations in 51 countries. He was responsible for overseeing all revenue generating business segments including Clinical Research Services, Calyx, and PAREXEL Consulting as well as sales, marketing, corporate quality, and information technology. Dr. Goldberg helped to pioneer PAREXEL’s strategic partnering approach with some of the world’s leading pharmaceutical companies and to build out the company’s global infrastructure, particularly in the Asia Pacific region, through both organic growth and acquisitions. Earlier in his PAREXEL career, he founded the company’s Medical Imaging business and helped establish its technology subsidiary, Perceptive Informatics (now PAREXEL Informatics). Dr. Goldberg holds a BS degree in computer science from MIT and an MD from the University of Massachusetts Medical School. He completed residency training in Radiology at Massachusetts General Hospital, where he also served as Chief Resident and a staff physician with academic appointments at Harvard Medical School. We believe that Dr. Goldberg’s medical background and public company board experience allows him to make valuable contributions to our Board.
Daniel J. Donovan joined the Board in January 2023. Mr. Daniel Donovan is an entrepreneur with extensive experience within the biotech industry. Since 2014 to present he has been the Chief Executive Officer of rareLife Solutions, Inc., a company creating the connections to engage, unify, and amplify the voices of patients, advocates, and caregivers to inform and accelerate the development and commercialization of emerging treatments especially in rare diseases. Dan was a member of the Board of Directors and Chief Business Officer at Cancer Prevention Pharmaceuticals (CPP), a late-stage pharmaceutical development company with compounds targeted at several rare diseases. Prior to rareLife and CPP, Dan established Envision Pharma in 2001, serving as President through June 2011. He was the visionary behind the creation and development of Datavision, the market leader in medical publications technology. Envision Pharma was acquired by the United BioSource Corporation (UBC) in April 2008. At UBC Mr. Daniel Donovan was Senior Vice President Strategy and Market Development. Dan began his career at Pfizer serving in a variety of positions of increasing responsibility, ranging from sales to market research and marketing in the US domestic and international market place, culminating in his position as Director and European Team Leader. During his time at Pfizer, he played a pivotal role in the commercialization of some of the pharmaceutical industry’s most successful product launches. Dan earned a Bachelor of Science degree in Finance at Lehigh University. We believe that Mr. Daniel Donovan’s background in cancer and rare disease, finance, drug development, patient advocacy and small company board experience allows him to make valuable contributions to our Board.
Family Relationships
There are no family relationships between any of our executive officers and directors.
Code of Business Conduct
Our board of directors established a Code of Conduct applicable to our directors, officers and employees. The Code of Conduct is accessible on our website at www.intensitytherapeutics.com. If we make any substantive amendments to the Code of Conduct or grant any waiver, including any implicit waiver, from a provision of the Code of Conduct to our officers, we will disclose the nature of such amendment or waiver on that website or in a report on Form 8-K.
Board Composition and Election of Directors
Our business and affairs are managed under the direction of our board of directors. The primary responsibilities of our board of directors are to provide oversight, strategic guidance, counseling and direction to our management. Our board of directors meets on a regular basis and additionally as required.
The number of directors is fixed by our board of directors, subject to the terms of our amended and restated certificate of incorporation and our second amended and restated bylaws. Our board of directors consists of four (4) directors, three (3) of whom qualify as “independent” under Nasdaq listing standards.
Directors are (except for the filling of vacancies and newly created directorships) elected by the holders of a plurality of the votes cast by the holders of shares present in person or represented by proxy at the meeting and entitled to vote on the election of such directors. In accordance with our amended and restated certificate of incorporation and our second amended and restated bylaws, our board of directors is divided into three classes with staggered three-year terms. Only one class of directors is elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Our directors are divided among the three classes as follows:
•the Class I director is Mr. Daniel Donovan, and his term will expire at the 2024 annual meeting of stockholders;
•the Class II director is Dr. Mark A. Goldberg, and his term will expire at the 2025 annual meeting of stockholders; and
•the Class III directors are Dr. Emer Leahy and Lewis H. Bender, and their terms will expire at the 2026 annual meeting of stockholders.
Each director’s term will continue until the election and qualification of his or her successor, or his or her earlier death, resignation or removal. Any increase or decrease in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors. This classification of our board of directors may have the effect of delaying or preventing changes in control of our company.
Director Independence
Our board of directors has undertaken a review of the independence of each director. Based on information provided by each director concerning his or her background, employment and affiliations, our board of directors has determined that Mr. Daniel Donovan, Dr. Emer Leahy and Dr. Mark A. Goldberg do not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is “independent” as that term is defined under the applicable rules and regulations of the SEC and the listing standards of Nasdaq. In making these determinations, our board of directors considered the current and prior relationships that each non-employee director has with our company and all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director, and the transactions involving them described in the section titled “Certain Relationships and Related Party Transactions.”
Committees of the Board of Directors
Our board of directors has established an audit committee, a compensation committee and a nominating and corporate governance committee. The composition and responsibilities of each of the committees of our board of directors
is described below. Members will serve on these committees until their resignation or until as otherwise determined by our board of directors.
Audit Committee
Our audit committee consists of Mr. Daniel Donovan, Dr. Emer Leahy and Dr. Mark A. Goldberg, with Dr. Emer Leahy serving as Chairperson. The composition of our audit committee meets the requirements for independence under current Nasdaq listing standards and SEC rules and regulations. Each member of our audit committee meets the financial literacy requirements of Nasdaq listing standards. In addition, our board of directors has determined that Dr. Emer Leahy is an audit committee financial expert within the meaning of Item 407(d) of Regulation S-K under the Securities Act of 1933. Our audit committee, among other things:
•reviews our consolidated financial statements and our critical accounting policies and practices;
•selects a qualified firm to serve as the independent registered public accounting firm to audit our consolidated financial statements;
•helps to ensure the independence and performance of the independent registered public accounting firm;
•discusses the scope and results of the audit with the independent registered public accounting firm and reviews, with management and the independent registered public accounting firm, our interim and year-end results of operations;
•pre-approves all audit and all permissible non-audit services to be performed by the independent registered public accounting firm;
•oversees the performance of our internal audit function when established;
•reviews the adequacy of our internal controls;
•develops procedures for employees to submit concerns anonymously about questionable accounting or audit matters;
•reviews our policies on risk assessment and risk management; and
•reviews related party transactions.
Our audit committee operates under a written charter that satisfies the applicable rules of the SEC and the listing standards of Nasdaq.
Compensation Committee
Our compensation committee consists of Mr. Daniel Donovan, Dr. Emer Leahy and Dr. Mark A. Goldberg, with Mr. Daniel Donovan serving as Chairperson. The composition of our compensation committee meets the requirements for independence under Nasdaq listing standards and SEC rules and regulations. Each member of the compensation committee is also a non-employee director, as defined pursuant to Rule 16b-3 promulgated under the Exchange Act. The purpose of our compensation committee is to discharge the responsibilities of our board of directors relating to compensation of our executive officers. Our compensation committee, among other things:
•reviews, approves and determines, or makes recommendations to our board of directors regarding, the compensation of our executive officers;
•administers our stock and equity incentive plans;
•reviews and approves, or make recommendations to our board of directors regarding, incentive compensation and equity plans; and
•establishes and reviews general policies relating to compensation and benefits of our employees.
Our compensation committee operates under a written charter that satisfies the applicable rules of the SEC and the listing standards of Nasdaq.
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee consists of Mr. Daniel Donovan, and Dr. Mark A. Goldberg, with Dr. Mark A. Goldberg serving as Chairperson. The composition of our corporate governance committee meets the
requirements for independence under Nasdaq listing standards and SEC rules and regulations. Our nominating and corporate governance committee, among other things:
•identifies, evaluates and selects, or makes recommendations to our board of directors regarding, nominees for election to our board of directors and its committees;
•evaluates the performance of our board of directors and of individual directors;
•considers and makes recommendations to our board of directors regarding the composition of our board of directors and its committees;
•reviews developments in corporate governance practices;
•oversees environmental, social and governance (ESG) matters;
•evaluates the adequacy of our corporate governance practices and reporting; and
•develops and makes recommendations to our board of directors regarding corporate governance guidelines and matters.
The nominating and corporate governance committee operates under a written charter that satisfies the applicable listing requirements and rules of Nasdaq.
Role of Board of Directors in Risk Oversight Process
Our board of directors has responsibility for the oversight of our risk management processes and, either as a whole or through its committees, regularly discusses with management our major risk exposures, their potential impact on our business and the steps we take to manage them. The risk oversight process includes receiving regular reports from board committees and members of senior management to enable our board of directors to understand our risk identification, risk management and risk mitigation strategies with respect to areas of potential material risk, including operations, finance, legal, regulatory, cybersecurity, strategic and reputational risk.
Item 11. Executive Compensation
The following table presents information regarding the total compensation awarded to, earned by, or paid to our chief executive officer and the two most highly compensated executive officers who were serving as executive officers as of December 31, 2023 for services rendered in all capacities to us for the years ended December 31, 2023 and 2022. These individuals are our named executive officers (“NEOs”) for 2023.
Summary Compensation Table
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name and Principal Position | | Year | | Salary ($) | | | Bonus ($) | | Stock Awards ($) | | Warrant and Option Awards ($)(1) | | Non-Equity Incentive Plan Compensation ($)(2) | | All Other Compensation ($)(3) | | | Total ($) |
| Lewis H. Bender | | 2023 | | 553,173 | | (4) | | — | | | — | | | — | | | 261,500 | | | 290,317 | | (5) | | 1,104,990 | |
| President and Chief Executive Officer | | 2022 | | 492,827 | | (4) | | — | | | — | | | 439,415 | | | — | | | 62,329 | | (6) | | 994,571 | |
| John Wesolowski | | 2023 | | 186,154 | | | | — | | | — | | | 254,920 | | | 67,015 | | | 19,202 | | (7) | | 527,291 | |
| Principal Accounting Officer and Controller | | 2022 | | 165,000 | | | | — | | | — | | | 81,207 | | | — | | | 4,950 | | (8) | | 251,157 | |
| James Ahlers | | 2023 | | — | | | | — | | | — | | | — | | | — | | | 75,088 | | (9) | | 75,088 | |
| Executive Vice President of Corporate Finance | | 2022 | | — | | | | — | | | — | | | 41,226 | | | — | | | 120,800 | | (9) | | 162,026 | |
(1)In accordance with SEC rules, these columns reflect the aggregate grant date fair value of the option awards and stock awards granted during 2023 and 2022 computed in accordance with Financial Accounting Standard Board ASC Topic 718 for stock-based compensation transactions, or ASC 718. These amounts do not reflect the actual economic value that will be realized by the named executive officer upon the vesting of stock options, the exercise of stock options or the sale of shares of our Common Stock. For a discussion of how we calculate stock-based compensation expense, see the Notes to Financial Statements included in Part II, Item 8 of this Form 10-K.
(2)The 2023 amounts reported represent cash bonuses earned under our 2023 bonus plan based upon the achievement of company objectives for the year ended December 31, 2023, which will be paid in 2024.
(3)Information includes perquisite and personal benefit received by each NEO (excludes perquisites and other personal benefits whose aggregate is less than $10,000).
(4)The amounts reported reflect the deferral and payment of $30,173 in salary from 2022 to 2023.
(5)The amounts reported represent $239,383 of accrued vacation payout in cash, $41,034 of company-paid portion of health and dental insurance and $9,900 in matching 401(k) Plan contributions of up to 3% of eligible earnings up to Federal limits.
(6)The amounts reported represent $53,179 of company-paid portion of health and dental insurance and $9,150 in matching 401(k) Plan contributions of up to 3% of eligible earnings up to Federal limits.
(7)The amounts reported represent $13,406 of accrued vacation payout in cash and $5,796 in matching 401(k) Plan contributions of up to 3% of eligible earnings up to Federal limits.
(8)Consists entirely of matching 401(k) Plan contributions of up to 3% of eligible earnings up to Federal limits.
(9)Consists entirely of consulting fees paid to and accrued to Danforth Advisors LLC.
Narrative Disclosure to Summary Compensation Table
Annual Base Salary
Our NEOs each receive a base salary to compensate them for services rendered to our company. The base salary payable to each named executive officer is intended to provide a fixed component of compensation reflecting the executive’s skill set, experience, role and responsibilities. Base salaries are reviewed annually, typically in connection with our annual performance review process, approved by our board of directors or the compensation committee, and may be adjusted from time to time to realign salaries with market levels after taking into account individual responsibilities, performance, and experience.
For fiscal year 2023, the annual base salaries for each of Mr. Bender and Mr. Wesolowski were $523,000 and $215,000 respectively. For fiscal year 2022, the annual base salaries for each of Mr. Bender and Mr. Wesolowski were $523,000 and $165,000, respectively. Mr. Ahlers is an independent consultant whose compensation for 2023 and 2022 was $75,088 and $120,800, respectively, at a current rate of $416 per hour.
Employment Agreements with our Named Executive Officers
Employment Agreement with Lew Bender
We have entered into an Amended and Restated Employment Agreement with Mr. Bender in connection with our IPO (the “Amended and Restated Employment Agreement”), which agreement became effective on November 29, 2021.
The Amended and Restated Employment Agreement provides that Mr. Bender will receive a base salary of $523,000, which will be reviewed annually and may be increased, but not decreased, without Mr. Bender’s consent. The Amended and Restated Employment Agreement also provides that Mr. Bender is eligible to receive an annual performance-based cash bonus as a percentage (not more than 75%) of base salary, which bonus is earned based on the achievement of performance targets, as determined annually by the Compensation Committee of our board of directors. Any annual bonus, to the extent earned, is paid in a lump sum. Under the Amended and Restated Employment Agreement, Mr. Bender is also eligible to participate in the Company’s equity grant program, which grants shall occur not less than once per year. The form of equity award agreement and the terms and conditions of such equity awards, including with respect to vesting, will be determined by our board of directors.
Under the Amended and Restated Employment Agreement, Mr. Bender may terminate his employment at any time and for any reason with prior notice. We may terminate Mr. Bender’s employment immediately upon his death, upon a period of disability or immediately upon written notice for “cause” (as defined below). In the event that Mr. Bender’s employment is terminated due to his death or disability, for “cause” or upon his resignation without “good reason” (as defined below), we must provide him (or his beneficiaries) with (i) any unpaid base salary through the date of termination, (ii) payment for any accrued but unused paid time off, (iii) reimbursement for expenses properly incurred, and (iv) all other vested entitlements or benefits to which he is entitled (collectively, the “Accrued Benefits”).
If we terminate the executive’s employment without cause or Mr. Bender terminates his employment for “good reason” (as defined below), then we must provide Mr. Bender with the Accrued Benefits and subject to his execution and non-revocation of a release of claims, a lump sum payment equal to two times the sum of (i) his annual base salary, plus (ii) his target annual bonus, in each case at the rates and target amounts in effect as of such termination of employment. If we terminate the executive’s employment without cause or Mr. Bender terminates his employment for good reason and such termination is concurrent with or within six months after a change of control of the Company, then in addition to
receiving the Accrued Benefits, but in lieu of other severance payments, Mr. Bender shall receive as a lump sum severance payment, at the time of such termination, an amount equal to (i) two and one-half (2.5) times the sum of (A) his base salary and (B) target annual bonus, each as in effect at the time of such termination, plus (ii) a payment equal to his target annual bonus for the calendar year in which the termination date occurred pro-rated for the period for which Mr. Bender was employed by us during such year.
For purposes of the Amended and Restated Employment Agreement, “cause” generally means the executive’s (i) the failure by the executive to cure a breach of a material duty imposed on the executive under the Amended and Restated Employment Agreement or any other written agreement between executive and the Company, or any policy of the Company, after written notice thereof by the Company, if curable in the reasonable discretion of the Board, (ii) acts by executive of fraud, embezzlement, theft, willful misconduct, gross negligence, or other material dishonesty directed against the Company, (iii) the failure or refusal by executive to perform any material duties under the Amended and Restated Employment Agreement or to follow any lawful and reasonable direction of the Company; or (vi) the executive’s being charged with a felony (other than a traffic offense), or a crime involving moral turpitude.
For purposes of the Amended and Restated Employment Agreement, “good reason” generally means a resignation by the executive on account of: (i) a material reduction in the executive’s duties, authority or responsibilities; (ii) relocation of executive’s place of employment without executive’s consent to a location more than fifty miles from the Company’s current executive offices; or (iii) any material breach by the Company of the Amended and Restated Employment Agreement. Good reason will not exist unless the executive notifies the Company in writing of such action not later than a set time after its initial occurrence and the Company has not remediated the action within a set time after such notice.
Employment Agreement with John Wesolowski
On June 20, 2023, we entered into an employment agreement with John Wesolowski (the “Wesolowski Employment Agreement”), pursuant to which he will serve as Interim Chief Financial Officer, Principal Accounting Officer and Controller of the Company. Under the Wesolowski Employment Agreement, Mr. Wesolowski is entitled to a base salary of $165,000. The Wesolowski Employment Agreement provides for at-will employment. Under the Wesolowski Employment Agreement, Mr. Wesolowski is also eligible to participate in the Company’s equity grant program. The form of equity award agreement and the terms and conditions of such equity awards, including with respect to vesting, will be determined by our board of directors. Effective July 22, 2023, Mr. Wesolowski’s base salary is $215,000.
The Wesolowski Employment Agreement also includes customary confidentiality and non-disparagement provisions, as well as provisions relating to assignment of inventions. The Wesolowski Employment Agreement also includes non-competition and non-solicitation of employees and customers provision that applies during the executive’s employment with the Company and for a period of one year after termination of employment.
Consulting Agreement with James Ahlers
On August 10, 2021, we entered into a Consulting Agreement (the “Consulting Agreement”) with Danforth Advisors, LLC, a company that provides strategic and operational finance and accounting services to life science companies, through which we retained the services of James Ahlers to initially serve as our Chief Financial Officer, and then eventually as our Vice President of Corporate Finance.
Pursuant to the Consulting Agreement, Mr. Ahlers performs services for the Company on a part-time basis and is compensated at an hourly rate. Mr. Ahler’s current hourly rate is $416 per hour. The term of the Consulting Agreement is to continue until either party shall give notice of termination, subject to the terms in the Agreement.
The Consulting Agreement also includes customary confidentiality and non-solicitation provisions, as well as provisions relating to assignment of inventions.
All Other Compensation
All other compensation includes: 1) medical and dental insurance; and 2) 401(k) plan matching contribution reflecting 3% of eligible earnings.
Outstanding Equity Awards at Fiscal Year End
The following table presents the outstanding equity awards held by each of our named executive officers as of December 31, 2023, as adjusted for the Reverse Split:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Option Awards |
| Name | | Number of securities underlying unexercised options exercisable (#) | | Number of securities underlying unexercised options
unexercisable (#) | | Option exercise price ($) | | Option expiration date |
| Lewis H. Bender | | 75,000 | | - | | 9.00 | | | 8/6/2029 |
| | 56,250 | | 18,750 | | 11.50 | | | 7/31/2030 |
| | 56,250 | | 18,750 | | 11.50 | | | 8/13/2031 |
| | 37,500 | | 37,500 | | 9.00 | | | 12/13/2032 |
| | | | | | | | |
| | | | | | | | |
| John Wesolowski | | 15,000 | | - | | 4.00 | | | 3/27/2027 |
| | 7,500 | | - | | 8.00 | | | 2/6/2028 |
| | 2,500 | | - | | 9.00 | | | 7/11/2029 |
| | 4,688 | | 1,563 | | 11.50 | | | 7/31/2030 |
| | 3,000 | | 3,000 | | 11.50 | | | 8/13/2031 |
| | 3,250 | | 3,250 | | 11.50 | | | 9/5/2031 |
| | 3,125 | | 9,375 | | 9.00 | | | 12/13/2032 |
| | 12,500 | | 37,500 | | 6.43 | | | 7/19/2033 |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Warrant Awards |
Name | | Number of securities underlying unexercised warrants exercisable (#) | | Number of securities underlying unexercised warrants
unexercisable (#) | | Warrant exercise price ($) | | Warrant expiration date |
James Ahlers | | 1,250 | | 3,750 | | 9.00 | | | 12/31/32 |
2013 Stock and Option Plan
Under our 2013 Stock and Option Plan, or the 2013 Plan, 4,500,000 shares of Common Stock have been reserved for issuance in the form of incentive stock options, non-qualified stock options, restricted stock, unrestricted stock, stock appreciation rights or any combination of the foregoing. The shares issuable pursuant to awards granted under the 2013 Plan are authorized but unissued shares.
The 2013 Plan is administered by our board or at the discretion of the board, which has full power to select the individuals to whom awards will be granted and to determine the specific terms and conditions of each award, subject to the provisions of the 2013 Plan. Pursuant to the 2013 Plan and subject to applicable law, our board of directors has delegated to the compensation committee the power to make recommendations to the board of directors relating to management compensation, the adoption of employee benefit plans, stock option or equity incentive plans and other similar matters.
The option exercise price of each option granted under the 2013 Plan is determined by our board of directors and may not be less than the fair market value of a share of Common Stock on the date of grant. The term of each option is fixed by the board and may not exceed 10 years from the date of grant. The board determines at what time or times each option may be exercised when granting the option.
The 2013 Plan provides that, upon the consummation of a sale event, unless provision is made in connection with the sale event for the assumption or continuation of the awards by the successor entity or substitution of the awards with new awards of the successor entity, with appropriate adjustment, the 2013 Plan and all outstanding and unexercised options
issued thereunder will terminate upon the effective time of the sale event. We may make or provide for cash payment to holders of options equal to the difference between (i) the per share cash consideration in the sale event multiplied by the number of shares subject to outstanding options being cancelled, and (ii) the aggregate exercise price to the holders of all vested and exercisable options.
Our board of directors may amend the 2013 Plan but no such action may adversely affect the rights of an award holder without such holder’s consent. Approval by our stockholders of amendments to the 2013 Plan must be obtained if required by law.
As of December 31, 2023, our board of directors has determined not to make any further awards under the 2013 Plan.
2021 Stock Incentive Plan
On November 12, 2021, we adopted a new equity incentive plan, the 2021 Stock Incentive Plan, or the 2021 Plan. Under the 2021 Plan, we may grant cash and equity incentive awards to eligible service providers in order to attract, motivate and retain the talent for which we compete. The material terms of the 2021 Plan are summarized below.
Types of Awards. The 2021 Plan provides for the grant of non-qualified stock options (“NQSOs”), incentive stock options (“ISOs”), restricted stock awards, restricted stock units (“RSUs”), unrestricted stock awards, stock appreciation rights and other forms of stock based compensation.
Eligibility and Administration. Employees, officers, consultants, directors, and other service providers of the Company and its affiliates are eligible to receive awards under the 2021 Plan. The 2021 Plan is administered by the board with respect to awards to non-employee directors and by the Compensation Committee with respect to other participants, each of which may delegate its duties and responsibilities to committees of the company’s directors and/or officers (all such bodies and delegates referred to collectively as the plan administrator), subject to certain limitations that may be imposed under Section 16 of the Exchange Act, and/or other applicable law or stock exchange rules, as applicable. The plan administrator has the authority to make all determinations and interpretations under, prescribe all forms for use with, and adopt rules for the administration of, the 2021 Plan, subject to its express terms and conditions. The plan administrator also sets the terms and conditions of all awards under the 2021 Plan, including any vesting and vesting acceleration conditions.
Share Reserve. Pursuant to the 2021 Plan, we have reserved 3,000,000 shares of the Common Stock for issuance thereunder, which reserve shall be increased annually beginning on January 1, 2022 and ending on and including January 1, 2031, equal to the lesser of (A) 3.5% of the aggregate number of shares of Common Stock outstanding on the final day of the immediately preceding calendar year or (B) such smaller number of shares as is determined by our board. The share reserve is subject to the following adjustments:
•The share limit is increased by the number of shares subject to awards granted that later are forfeited, expire or otherwise terminate without issuance of shares, or that are settled for cash or otherwise do not result in the issuance of shares.
•Shares that are withheld upon exercise to pay the exercise price of a stock option or satisfy any tax withholding requirements are added back to the share reserve and again are available for issuance under the 2021 Plan.
Pursuant to the provisions of the 2021 Plan, the authorized shares were increased from 3,000,000 to 3,238,700 effective January 1, 2023. As of December 31, 2023, options to purchase 2,837,700 shares of Common Stock were available to be issued under the 2021 Plan. On January 1, 2024, pursuant to the provisions of the 2021 Plan, authorized shares increased by 479,828 shares.
Awards issued in substitution for awards previously granted by a company that merges with, or is acquired by, the Company do not reduce the share reserve limit under the 2021 Plan.
Director Compensation. The 2021 Plan provides for an annual limit on non-employee director compensation of $500,000, increased to $750,000 in the fiscal year of a non-employee director’s initial service as a non-employee member of the board of directors of the Company. This limit applies to the sum of both equity grants that could be awarded to non-employee directors during a fiscal year (based on their value under ASC Topic 718 on the grant date) and cash compensation, such as cash retainers and meeting fees earned during a fiscal year. Notwithstanding the foregoing, the board reserves the right to make an exception to these limits due to extraordinary circumstances without the participation of the affected director receiving the additional compensation.
Stock Options. ISOs may be granted only to employees of the Company, or to employees of a parent or subsidiary of the Company, determined as of the date of grant of such options. An ISO granted to a prospective employee upon the condition that such person becomes an employee shall be deemed granted effective on the date such person commences
employment. The exercise price of an ISO shall not be less than 100% of the fair market value of the shares covered by the awards on the date of grant of such option or such other price as may be determined pursuant to the Internal Revenue Code of 1986, as amended from time to time (the “Code”). Notwithstanding the foregoing, an ISO may be granted with an exercise price lower than the minimum exercise price set forth above if such award is granted pursuant to an assumption or substitution for another option in a manner that complies with the provisions of Section 424(a) of the Code. Notwithstanding any other provision of the 2021 Plan to the contrary, no ISO may be granted under the 2021 Plan after 10 years from the date that the 2021 Plan was adopted. No ISO shall be exercisable after the expiration of 10 years after the effective date of grant of such award, subject to the following sentence. In the case of an ISO granted to a ten percent stockholder, (i) the exercise price shall not be less than 110% of the fair market value of a share on the date of grant of such ISO, and (ii) the exercise period shall not exceed 5 years from the effective date of grant of such ISO.
Restricted Stock and Restricted Stock Units. The committee may award restricted stock and RSUs under the 2021 Plan. Restricted stock awards consist of shares of stock that are transferred to the participant subject to restrictions that may result in forfeiture if specified vesting conditions are not satisfied. RSU awards result in the transfer of shares of stock to the participant only after specified vesting conditions are satisfied. A holder of restricted stock is treated as a current stockholder and shall be entitled to dividend and voting rights, whereas the holder of a restricted stock unit is treated as a stockholder with respect to the award only when the shares are delivered in the future. RSUs may include dividend equivalents. Specified vesting conditions may include performance goals to be achieved during any performance period and the length of the performance period. The committee may, in its discretion, make adjustments to performance goals based on certain changes in the Company’s business operations, corporate or capital structure or other circumstances. When the participant satisfies the conditions of an RSU award, the Company may settle the award (including any related dividend equivalent rights) in shares, cash or other property, as determined by the committee, in its sole discretion.
Other Shares or Share-Based Awards. The committee may grant other forms of equity-based or equity-related awards other than stock options, restricted stock or restricted stock units. The terms and conditions of each stock-based award shall be determined by the committee.
Clawback Rights. Awards granted under the 2021 Plan will be subject to recoupment or clawback under the Company’s clawback policy or applicable law, both as in effect from time to time.
Sale of the Company. Awards granted under the 2021 Plan automatically accelerate and vest, become exercisable (with respect to stock options), or have performance targets deemed earned at target level if there is a sale of the Company. The Company does not use a “liberal” definition of change in control as defined in Institutional Shareholder Services’ proxy voting guidelines.
No Repricing. The 2021 Plan prohibits the amendment of the terms of any outstanding award, and any other action taken in a manner to achieve (i) the reduction of the exercise price of NQSOs, ISOs or stock appreciation rights (collectively, “Stock Rights”); (ii) the cancellation of outstanding Stock Rights in exchange for cash or other awards with an exercise price that is less than the exercise price or base price of the original award; (iii) the cancellation of outstanding Stock Rights with an exercise price or base price that is less than the then current fair market value of a share of Common Stock in exchange for other awards, cash or other property; or (iv) otherwise effect a transaction that would be considered a “repricing” for the purposes of the stockholder approval rules of the applicable securities exchange or inter-dealer quotation system on which the Common Stock is listed or quoted without stockholder approval.
Transferability of Awards. Except as described below, awards under the 2021 Plan generally are not transferable by the recipient other than by will or the laws of descent and distribution. Any amounts payable or shares issuable pursuant to an award generally will be paid only to the recipient or the recipient’s beneficiary or representative. The committee has discretion, however, to permit certain transfer of awards to other persons or entities.
Adjustments. As is customary in incentive plans of this nature, each share limit and the number and kind of shares available under the 2021 Plan and any outstanding awards, as well as the exercise price or base price of awards, and performance targets under certain types of performance-based awards, are subject to adjustment in the event of certain reorganizations, mergers, combinations, recapitalizations, stock splits, stock dividends, or other similar events that change the number or kind of shares outstanding, and extraordinary dividends or distributions of property to the stockholders. The number of shares available under the 2021 Plan was not adjusted as part of the Reverse Split.
Amendment and Termination. The board of directors may amend, modify or terminate the 2021 Plan without stockholder approval, except that stockholder approval must be obtained for any amendment that, in the reasonable opinion of the board or the committee, constitute a material change requiring stockholder approval under applicable laws, policies or regulations or the applicable listing or other requirements of a stock exchange on which shares of Common Stock are then listed. The 2021 Plan will terminate upon the earliest of (1) termination of the 2021 Plan by the board of directors, or
(2) the tenth anniversary of the board adoption of the 2021 Plan. Awards outstanding upon expiration of the 2021 Plan shall remain in effect until they have been exercised or terminated, or have expired.
Director Compensation
The following table provides certain information concerning compensation for each person who served as a non-employee member of our board of directors for the year ended December 31, 2023. Other than as set forth in the table and described more fully below, we did not make equity awards or pay any other compensation to any non-employee members of our board of directors in 2023. During fiscal year 2023, Lewis H. Bender, our President and Chief Executive Officer, served as a member of our board of directors and received no additional compensation for his services as a member of our board of directors. See the section titles “Executive Compensation” in Item 11 for more information about Mr. Bender’s compensation for fiscal year 2023. We reimburse non-employee members of our board of directors for reasonable travel and out-of-pocket expenses incurred in attending meetings of our board of directors and committees of our board of directors. All fees under the director compensation policy are paid on a quarterly basis in arrears and no meeting fees are paid.
| | | | | | | | | | | | | |
| Name | Fees Earned or
Paid in Cash
($) | | | | Total
($) |
| Dr. Emer Leahy | 31,750 | | | | | 31,750 | |
| Dr. Mark A. Goldberg | 26,750 | | | | | 26,750 | |
| Mr. Daniel Donovan | 28,750 | | | | | 28,750 | |
Non-Employee Director Compensation Policy
Our board of directors has adopted a non-employee director compensation policy that is designed to enable us to attract and retain, on a long-term basis, highly qualified non-employee directors. Under the policy, each director who is not an employee will be paid cash compensation as set forth below:
| | | | | |
| ANNUAL RETAINER |
Board of Directors: | |
All non-employee members | $ | 40,000 | |
Audit Committee: | |
Chair | $ | 20,000 | |
Members | $ | 10,000 | |
Compensation Committee: | |
Chair | $ | 15,000 | |
Members | $ | 7,000 | |
Nominating and Corporate Governance Committee: | |
Chair | $ | 10,000 | |
Members | $ | 5,000 | |
Compensation Committee Interlocks and Insider Participation
The Compensation Committee of the Board of Directors is currently composed of the following three non-employee directors: Dr. Emer Leahy, Dr. Mark Goldberg, and Mr. Daniel Donovan. No member of the Compensation Committee is or was formerly an officer or employee of the Company during the last fiscal year. In addition, no executive officer of the Company serves on the compensation committee or board of directors of a company for which any of the Company’s directors serve as an executive officer. See Item 13.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table sets forth the number of shares of common stock beneficially owned as of March 1, 2024 by:
•each of our stockholders who is known by us to beneficially own 5% or more of our common stock;
•each of our named executive officers;
•each of our directors; and
•all of our directors and current executive officers as a group.
Beneficial ownership is determined based on the rules and regulations of the SEC. A person has beneficial ownership of shares if such individual has the power to vote and/or dispose of shares. This power may be sole or shared and direct or indirect. Applicable percentage ownership in the following table is based on 13,709,377 shares outstanding as of March 1, 2024. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of common stock that are subject to options or warrants held by that person and exercisable as of, or within 60 days of March 1, 2024 are counted as outstanding. These shares, however, are not counted as outstanding for the purposes of computing the percentage ownership of any other person(s). Except as may be indicated in the footnotes to this table and pursuant to applicable community property laws, each person named in the table has sole voting and dispositive power with respect to the shares of common stock set forth opposite that person’s name. Unless indicated below, the address of each individual listed below is c/o Intensity Therapeutics Inc., 1 Enterprise Drive, Suite 430, Shelton, CT 06484-4779.
| | | | | | | | | | | | | | |
| Name of Beneficial Owner | | Number of Shares
Beneficially Owned | | Percentage of Shares
Beneficially Owned |
| | | | |
| Directors and Executive Officers | | | | |
Lewis H. Bender(1) | | 2,245,000 | | 16.1 | % |
| | | | |
John Wesolowski(2) | | 57,254 | | * |
James Ahlers(3) | | 1,250 | | * |
Dr. Emer Leahy(4) | | 79,000 | | * |
Dr. Mark A. Goldberg(5) | | 69,000 | | * |
Mr. Daniel Donovan(6) | | 12,500 | | * |
Directors and Executive Officers as a group (7 persons)(7) | | 2,464,004 | | 17.4 | % |
| | | | |
| 5% Stockholders | | | | |
Leonard Batterson(8) | | 2,476,213 | | 18.0 | % |
Larry Levy(9) | | 885,764 | | 6.4 | % |
Craig J. Duchossois(10) | | 828,069 | | 6.0 | % |
| | | | |
| * Less than 1% | | | | |
(1)Includes 225,000 shares of common stock issuable upon the exercise of options that are exercisable within sixty days of March 1, 2024. Does not include 75,000 shares of common stock underlying options that are not exercisable within sixty days of March 1, 2024.
(2)Includes 51,563 shares of common stock issuable upon the exercise of options that are exercisable within sixty days of March 1, 2024. Does not include 54,687 shares of common stock underlying options that are not exercisable within sixty days of March 1, 2024.
(3)Includes 1,250 shares of common stock issuable upon the exercise of warrants that are exercisable within sixty days of March 1, 2024. Does not include 3,750 shares of common stock underlying warrants that are not exercisable within sixth days of March 1, 2024.
(4)Includes 79,000 shares of common stock issuable upon the exercise of options that are exercisable within sixty days of March 1, 2024. Does not include 18,750 shares of common stock underlying options that are not exercisable within sixty days of March 1, 2024.
(5)Includes 69,000 shares of common stock issuable upon the exercise of options that are exercisable within sixty days of March 1, 2024. Does not include 18,750 shares of common stock underlying options that are not exercisable within sixty days of March 1, 2024.
(6)Includes 12,500 shares of common stock issuable upon the exercise of options that are exercisable within sixty days of March 1, 2024. Does not include 12,500 shares of common stock underlying options that are not exercisable within sixty days of March 1, 2024.
(7)Includes 438,313 shares of common stock issuable upon the exercise of options that are exercisable within sixty days of March 1, 2024. Does not include 263,437 shares of common stock underlying options that are not exercisable within sixty days of March 1, 2024.
(8)Consists of (i) 2,023,227 shares of Common Stock held by VCapital Intensity LLC, (ii) 427,986 shares of Common Stock held by BVC — Intensity LLC and (iii) 25,000 shares of Common Stock issuable upon the exercise of warrants exercisable within 60 days after March 1, 2024. Batterson may be deemed to beneficially own such shares. The principal business address of VCapital Intensity LLC and BVC — Intensity LLC is 901 W. Jackson Blvd., Suite 503 Chicago, IL 60607.
(9)Consists of (i) 387,500 shares of Common Stock held by LFP River West Investors, LLC — Series 21, (ii) 370,531 shares of Common Stock held by LFP River West Investors, LLC — Series 38 and (iii) 75,733 shares of Common Stock held by Levy Family Investors LLC and (iv) 52,000 shares of Common Stock issuable upon the exercise of warrants exercisable within 60 days after March 1, 2024. Does not include 24,000 shares of common stock underlying options that are not exercisable within sixty days of March 1, 2024. Mr. Levy may be deemed to beneficially own such shares. The registered address for LFP River West Investors, LLC is 251 Little Falls Drive, Wilmington, DE 19808.
(10)Consists of (i) 812,069 shares of Common Stock and (ii) 16,000 shares of Common Stock issuable upon the exercise of warrants exercisable within 60 days after March 1, 2024. Does not include 24,000 shares of common stock underlying options that are not exercisable within sixty days of March 1, 2024. All shares are held by Craig J. Duchossois Revocable Trust UAD 9/11/1989. Mr. Duchossois may be deemed to beneficially own such shares. The principal business address of Craig J. Duchossois is 444 W. Lake St, Suite 2000, Chicago, Illinois 60606.
The following table summarizes information about our equity compensation plans as of December 31, 2023.
| | | | | | | | | | | | | | | | | | | | |
| Plan Category | | Number of Shares of
Common Stock to be
Issued upon Exercise of
Outstanding Options (1) | | Weighted-Average
Exercise Price of
Outstanding Options | | Number of
Options
Remaining
Available for
Future Issuance
Under Equity
Compensation
Plans (excluding
securities
reflected in
column (a))(2) |
| | (a) | | (b) | | (c) |
| Equity compensation plans approved by stockholders | | 1,239,750 | | $ | 8.00 | | | 2,837,700 |
| Equity compensation plans not approved by stockholders | | - | | - | | | - |
| Total | | 1,239,750 | | $ | 8.00 | | | 2,837,700 |
(1)The amounts shown in this column include securities under both the 2013 Plan and 2021 Plan.
(2)Consists entirely of securities under the 2021 Plan. In accordance with the provisions in our 2021 Plan, the Board authorized that an additional 479,828 shares would become available for issuance on January 1, 2024, which represents approximately 3% of the shares outstanding on December 31, 2023. These shares are excluded from this calculation.
Item 13. Certain Relationships and Related Transactions, and Director Independence
In addition to the compensation arrangements, including employment, termination of employment and change in control arrangements and indemnification arrangements, discussed, when required, in the sections titled “Management” and “Executive Compensation,” the following is a description of each transaction since January 1, 2022 and each currently proposed transaction in which:
•we have been or are to be a participant;
•the amount involved exceeded or exceeds the lesser of $120,000 or 1% of our assets; and
•any of our directors, executive officers or holders of more than 5% of our capital stock, or any immediate family member of, or person sharing the household with, any of these individuals, had or will have a direct or indirect material interest.
Convertible Note with Shareholder
On September 20, 2021, we entered into a convertible debt agreement (the “2021 Convertible Note”) for aggregate principal of $2,000,000. On November 29, 2022 and again February 8, 2023, we amended the 2021 Convertible Note to reflect new terms upon the Company’s IPO or equity financing (the “2021 Amended Note”). Pursuant to the terms of the 2021 Amended Note, the maturity date is October 1, 2025, and has the following conversion terms. The outstanding principal balance together with the unpaid and accrued interest of the note will be automatically converted upon the earliest of (i) an IPO in excess of $8,000,000 gross proceeds, (ii) a sale event of all or substantially all of the company’s assets or a majority of its equity securities, (iii) non-IPO financing by selling preferred stock in an equity offering other than an IPO or (iv) the maturity date of October 1, 2025. If an IPO, sale event or non-IPO financing occurs between November 29, 2022 through March 20, 2023 a conversion price discount of 30% would be assessed, if between March 20, 2023 through October 1, 2025 a conversion price discount of 35% would be assessed. Otherwise at the maturity date a conversion price of $11.50 per share would be assessed. The 2021 Amended Note accrues interest at 3% per annum, but will increase to 6% per annum after October 1, 2023, and is convertible to shares of our Common Stock. The occurrence of any of the following shall constitute an event of default: a) failure to pay when due any principal payment; b) voluntary bankruptcy or insolvency proceedings; c) involuntary bankruptcy or insolvency proceedings; d) judgements in excess of $500,000; or e) defaults under other indebtedness. Under these occurrences, the holder may declare all outstanding principal and interest payable to be immediately due and payable. The 2021 Amended Note automatically converted upon the IPO into 648,109 shares of Common Stock.
On November 29, 2022, we entered into a convertible debt agreement (the “2022 Convertible Note”) for $1,500,000. On February 8, 2023, we amended the 2022 Convertible Note (the “2022 Convertible Note Amendment”) to reflect new terms upon the Company’s IPO or equity financing. The outstanding principal balance together with the unpaid and accrued interest of the note will be automatically converted upon the earliest of (i) an IPO of no less than $8,000,000 gross proceeds, (ii) a sale event of all or substantially all of the company’s assets or a majority of its equity securities, (iii) non-IPO financing by selling preferred stock in an equity offering other than an IPO or (iv) the maturity date of October 1, 2025. If an IPO, sale event or non-IPO financing occurs prior to October 1, 2025 a conversion price discount of 30% would be assessed. Otherwise at the maturity date a conversion price would be $11.50 per share be assessed. The 2022 Amended Note automatically converted upon the IPO into 453,463 shares of Common Stock.
On March 30, 2023, we entered into a convertible debt agreement (the “2023 Convertible Note”) for $155,000. The outstanding principal balance together with the unpaid and accrued interest would be automatically converted upon the earliest of (i) an IPO of no less than $7,000,000 in gross proceeds, (ii) a sale event of all or substantially all of the Company’s assets or a majority of its equity securities, (iii) non-IPO financing by selling preferred stock in an equity offering other than an IPO or (iv) the maturity date of March 30, 2026. If an IPO, sale event or non-IPO financing occurs prior to March 30, 2026 a conversion price discount of 30% would be assessed; otherwise at the maturity date a conversion price would be $11.50 per share would be assessed. This note converted into 45,389 shares of Common Stock at our IPO.
The 2021 Amended Note, the 2022 Convertible Note Amendment and the 2023 Convertible Note were entered into with Leonard Batterson, one of our 10% shareholders.
Indemnification Agreements
We entered into indemnification agreements with our directors and executive officers. The indemnification agreements provide for indemnification against expenses, judgments, fines and penalties actually and reasonably incurred by an indemnitee in connection with threatened, pending or completed actions, suits or other proceedings, subject to certain limitations. The indemnification agreements also provide for the advancement of expenses in connection with a proceeding prior to a final, non-appealable judgment or other adjudication, provided that the indemnitee provides an undertaking to repay to us any amounts advanced if the indemnitee is ultimately found not to been titled to indemnification by us. The indemnification agreement set forth procedures for making and responding to a request for indemnification or advancement of expenses, as well as dispute resolution procedures that apply to any dispute between us and an indemnitee arising under the Indemnification Agreements.
Policies and Procedures for Related Party Transactions
We have adopted a policy that our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of any class of our common stock, any members of the immediate family of any of the foregoing persons and any firms, corporations or other entities in which any of the foregoing persons is employed or is a partner or principal or in a similar position or in which such person has a 5% or greater beneficial ownership interest, which we refer to collectively as related parties, are not permitted to enter into a transaction with us without the prior consent of our board of directors acting through the audit committee or, in certain circumstances, the chairman of the audit committee. Any request for us to enter into a transaction with a related party, in which the amount involved exceeds $100,000 and such related party would have a direct or indirect interest must first be presented to our audit committee, or in certain circumstances the chairman of our audit committee, for review, consideration and approval. In approving or rejecting any such proposal, our audit committee, or the chairman of our audit committee, is to consider the material facts of the transaction, including, but not limited to, whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances, the extent of the benefits to us, the availability of other sources of comparable products or services and the extent of the related party’s interest in the transaction.
Director Independence
Based on information requested from and provided by each of our directors, our board of directors has determined that Messrs. Mark Goldberg and Daniel Donovan and Ms. Emer Leahy are “independent directors” as such term is defined in the rules of the Nasdaq’s corporate governance requirements and Rule 10A-3 promulgated under the Securities Exchange Act of 1934, as amended.
Item 14. Principal Accountant Fees and Services
The following table represents aggregate fees billed to the Company for fiscal years ended December 31, 2023 and 2022, by EisnerAmper LLP, the Company’s independent registered public accounting firm. Amounts are rounded to thousands.
| | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 |
| Audit Fees | $467,250 | | | $332,325 | |
| Audit-Related Fees | - | | | - | |
| Tax Fees | - | | | - | |
| All Other Fees | - | | | - | |
| Total Fees | 467,250 | | | 332,325 | |
Audit Fees consist of fees billed for professional services rendered for the audit of our annual financial statements, review of our interim financial statements, comfort and consent letters. Audit fees includes fees for consents and comfort letters of $195,000 in 2023 and $167,000 in 2022.
Audit-Related Fees consist of fees billed for professional services rendered for assurance related services that are reasonably related to the performance of the audit or review of our financial services.
Tax Fees are for tax-related services related primarily to tax consulting and planning.
All Other Fees consist of the aggregate fees billed for any other products and services provided by the principal accountants.
Pre-Approval Policies and Procedures
The Audit Committee pre-approves all auditing services and any non-audit services that the independent registered public accounting firm is permitted to render under Section10A (h) of the Exchange Act. The Audit Committee may delegate the pre-approval to one of its members, provided that if such delegation is made, the full Audit Committee must be presented at its next regularly scheduled meeting with any pre-approval decision made by that member.
PART IV
Item 15. Exhibits, Financial Statement Schedules
(a)The following documents are filed as part of this report:
(1)Financial Statements
See Index to Consolidated Financial Statements as Part II Item 8 “Financial Statements and Supplementary Data.”
(2)Financial Statement Schedules
The financial statement schedules are omitted as they are either not applicable or the information required is presented in the financial statements and notes thereto under Part II Item 8. “Financial Statements and Supplementary Data.”
| | | | | | | | |
| Exhibit No. | | Description |
| 3.1 | | |
| | |
| 3.2 | | |
| | |
| 4.1 | | |
| | |
| 4.2 | | |
| | |
| 10.1 | | |
| | |
| 10.2# | | |
| | |
| 10.3# | | |
| | |
| 10.4# | | |
| | |
| 10.5† | | |
| | |
| 10.6† | | |
| | |
| 10.7† | | |
| | |
| 10.8# | | |
| | |
| 10.9 | | |
| | |
| 10.10# | | |
| | |
| | | | | | | | |
| 10.11# | | |
| | |
| 21.1 | | |
| | |
| 23.1* | | |
| | |
| 31.1* | | |
| | |
31.2* | | |
| | |
| 32.1* | | |
| | |
| 32.2* | | |
| | |
| 97.1* | | |
| | |
| 101.INS* | | Inline XBRL Instance Document |
| | |
| 101.SCH* | | Inline XBRL Taxonomy Extension Schema Document. |
| | |
| 101.CAL* | | Inline XBRL Taxonomy Extension Calculation Linkbase Document. |
| | |
| 101.DEF* | | Inline XBRL Taxonomy Extension Definition Linkbase Document. |
| | |
| 101.LAB* | | Inline XBRL Taxonomy Extension Label Linkbase Document. |
| | |
| 101.PRE* | | Inline XBRL Taxonomy Extension Presentation Linkbase Document. |
| | |
| 104* | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
______________________________________
# Indicates a management contract or compensatory plan or arrangement.
†Certain information has been excluded from the exhibit because it both (i) is not material and (ii) would likely cause competitive harm to the Registrant if publicly disclosed.
*Filed herewith.
Item 16. Form 10-K Summary
None
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | |
| Intensity Therapeutics, Inc. |
| | |
Date: March 14, 2024 | By: | /s/ Lewis H. Bender |
| | Lewis H. Bender |
| | President, Chief Executive Officer and Chairman |
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this Report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| Name | | Title | | Date |
| | | | | |
| /s/ Lewis H. Bender | | President, Chief Executive Officer and Chairman | | March 14, 2024 |
| Lewis H. Bender | | (principal executive officer) | | |
| | | | |
| /s/ Joseph Talamo | | Chief Financial Officer | | March 14, 2024 |
| Joseph Talamo | | (principal financial officer) | | |
| | | | |
/s/ John Wesolowski | | Principal Accounting Officer and Controller | | March 14, 2024 |
| John Wesolowski | | (principal accounting officer) | | |
| | | | |
| /s/ Daniel Donovan | | Director | | March 14, 2024 |
| Daniel Donovan | | | | |
| | | | |
| /s/ Dr. Emer Leahy | | Director | | March 14, 2024 |
| Dr. Emer Leahy | | | | |
| | | | | |
| /s/ Dr. Mark A. Goldberg | | Director | | March 14, 2024 |
| Dr. Mark A. Goldberg | | | | |
INTENSITY THERAPEUTICS, INC.
INDEX TO FINANCIAL STATEMENTS
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Intensity Therapeutics, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Intensity Therapeutics, Inc. (the “Company”) as of December 31, 2023 and 2022, and the related statements of operations, changes in redeemable convertible preferred stock and stockholders’ equity (deficiency), and cash flows for each of the years then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has incurred losses from operations and negative cash flows that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
We have served as the Company’s auditor since 2017.
/s/ EisnerAmper LLP
EISNERAMPER LLP
New York, New York
March 14, 2024
INTENSITY THERAPEUTICS, INC.
BALANCE SHEETS
(in thousands, except share and per share amounts)
| | | | | | | | | | | |
| December 31, |
| 2023 | | 2022 |
| ASSETS | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 8,556 | | | $ | 1,312 | |
| Marketable debt securities | 6,220 | | | - | |
| Prepaid expenses | 682 | | | 63 | |
| Other current assets | 6 | | | 76 | |
| Total current assets | 15,464 | | | 1,451 | |
| Right-of-use asset, net | 147 | | | 139 | |
| Other assets | 1,684 | | | 167 | |
| Total assets | $ | 17,295 | | | $ | 1,757 | |
| | | |
| LIABILITIES, REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY (DEFICIENCY) | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 3,048 | | | $ | 603 | |
| Accrued expenses | 891 | | | 1,724 | |
| Lease liability, current portion | 20 | | | 143 | |
| Convertible note and accrued interest | - | | | 4,349 | |
| Total current liabilities | 3,959 | | | 6,819 | |
| Other long-term liabilities | 36 | | | 36 | |
| Lease liability, net of long-term portion | 138 | | | - | |
| Total liabilities | $ | 4,133 | | | $ | 6,855 | |
Series A redeemable convertible preferred stock, par value $.0001. Authorized, issued, and outstanding shares of none and 5,000,000 as of December 31, 2023 and 2022, respectively. | - | | | 10,000 | |
| Commitments and contingencies | | | |
| | | |
| STOCKHOLDERS’ EQUITY (DEFICIENCY) | | | |
| | | |
Preferred stock, par value $.0001. Authorized shares of 15,000,000 and 20,000,000 as of December 31, 2023 and 2022, respectively. | - | | | - | |
| | | |
Series B convertible preferred stock, par value $.0001. Designated, issued, and outstanding shares of none and 1,449,113 as of December 31, 2023 and 2022, respectively. | - | | | - | |
| | | |
Series C convertible preferred stock, par value $.0001. Designated, issued, and outstanding shares of none and 1,800,606 as of December 31, 2023 and 2022, respectively. | - | | | - | |
| | | |
Common stock, par value $.0001. Authorized shares of 135,000,000 and 50,000,000 as of December 31, 2023 and 2022, respectively. Issued and outstanding shares of 13,709,377 and 3,410,103 as of December 31, 2023 and 2022, respectively. | 1 | | | - | |
| Additional paid-in capital | 63,676 | | | 23,555 | |
| Accumulated deficit | (50,515) | | | (38,653) | |
| Total stockholders’ equity (deficiency) | $ | 13,162 | | | $ | (15,098) | |
| Total liabilities, redeemable convertible preferred stock and stockholders’ equity (deficiency) | $ | 17,295 | | | $ | 1,757 | |
The accompanying notes are an integral part of these financial statements.
INTENSITY THERAPEUTICS, INC.
STATEMENTS OF OPERATIONS
(in thousands, except share and per share amounts)
| | | | | | | | | | | | | | | |
| | | Years Ended December 31, |
| | | | | 2023 | | 2022 |
| Operating expenses: | | | | | | | |
| Research and development | | | | | $ | 4,786 | | | $ | 5,132 | |
| General and administrative | | | | | 3,533 | | | 2,418 | |
| Total operating expenses | | | | | 8,319 | | | 7,550 | |
| Loss from operations | | | | | (8,319) | | | (7,550) | |
| | | | | | | |
| Other income (expense): | | | | | | | |
| Interest income | | | | | 324 | | | 2 | |
| Interest expense | | | | | (305) | | | (82) | |
| Loss on debt extinguishment | | | | | (2,262) | | | - | |
| Other | | | | | 24 | | | 48 | |
| Net loss | | | | | $ | (10,538) | | | $ | (7,582) | |
| | | | | | | |
| Preferred stock deemed dividend | | | | | (1,324) | | | - | |
| Net loss attributable to common stockholders | | | | | $ | (11,862) | | | $ | (7,582) | |
| | | | | | | |
| Loss per share, basic and diluted | | | | | $ | (1.38) | | | $ | (2.22) | |
| Weighted average number of shares of common stock, basic and diluted. | | | | | 8,616,324 | | 3,410,103 |
The accompanying notes are an integral part of these financial statements.
INTENSITY THERAPEUTICS, INC.
STATEMENTS OF CHANGES IN REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY (DEFICIENCY)
(in thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Series A Redeemable Convertible Preferred Stock | | | Series B Convertible Preferred | | Series C Convertible Preferred | | Common Stock | | Additional Paid in Capital | | Accumulated Deficit | | Stockholders’ Equity (Deficiency) |
| Shares | | Amount | | | Shares | | Amount | | Shares | | Amount | | Shares | | Amount | | | |
| Balances at December 31, 2021 | 5,000,000 | | $ | 10,000 | | | | 1,449,113 | | $ | - | | | 1,800,606 | | $ | - | | | 3,410,103 | | $ | - | | | $ | 22,386 | | | $ | (31,071) | | | $ | (8,685) | |
| Stock-based compensation expense | - | | - | | | | - | | - | | | - | | - | | | - | | - | | | 1,169 | | | - | | | 1,169 | |
| Net loss | - | | - | | | | - | | - | | | - | | - | | | - | | - | | | - | | | (7,582) | | | (7,582) | |
| Balances at December 31, 2022 | 5,000,000 | | $ | 10,000 | | | | 1,449,113 | | $ | - | | | 1,800,606 | | $ | - | | | 3,410,103 | | $ | - | | | $ | 23,555 | | | $ | (38,653) | | | $ | (15,098) | |
Issuance of common stock in public offering for cash, net of $3,403 issuance costs | - | | - | | | | - | | - | | | - | | - | | | 4,485,000 | | 1 | | | 19,022 | | | - | | | 19,023 | |
| Warrants issued to underwriters in connection with public offering | - | | - | | | | - | | - | | | - | | - | | | - | | - | | | 1,170 | | | - | | | 1,170 | |
| Conversion of preferred stock into common stock | (5,000,000) | | (10,000) | | | | (1,449,113) | | - | | | (1,800,606) | | - | | | 4,124,851 | | - | | | 10,000 | | | - | | | 10,000 | |
| Conversion of convertible notes into common stock | - | | - | | | | - | | - | | | - | | - | | | 1,399,716 | | - | | | 6,998 | | | - | | | 6,998 | |
| Warrants issued to convertible note holders | - | | - | | | | - | | - | | | - | | - | | | - | | - | | | 159 | | | - | | | 159 | |
| Exercise of options and warrants | - | | - | | | | - | | - | | | - | | - | | | 25,000 | | - | | | 50 | | | - | | | 50 | |
| Deemed dividend | - | | - | | | | - | | - | | | - | | - | | | 264,707 | | - | | | 1,324 | | | (1,324) | | | - | |
| Stock-based compensation expense | - | | - | | | | - | | - | | | - | | - | | | - | | - | | | 1,398 | | | - | | | 1,398 | |
| Net loss | - | | - | | | | - | | - | | | - | | - | | | - | | - | | | | | (10,538) | | | (10,538) | |
| Balances at December 31, 2023 | - | | $ | - | | | | - | | $ | - | | | - | | $ | - | | | 13,709,377 | | $ | 1 | | | $ | 63,676 | | | $ | (50,515) | | | $ | 13,162 | |
The accompanying notes are an integral part of these financial statements.
INTENSITY THERAPEUTICS, INC.
STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | | |
| Years Ended December 31, |
| 2023 | | 2022 |
| Cash flows from operating activities: | | | |
| Net loss | (10,538) | | | (7,582) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | |
| Amortization of discount on convertible notes | 159 | | | - | |
| Change in carrying value of right-of-use asset | 147 | | | 179 | |
| Stock-based compensation expense | 1,398 | | | 1,169 | |
| Loss on debt extinguishment | 2,262 | | | - | |
| Changes in operating assets and liabilities, net: | | | |
| Accrued interest on marketable debt securities | (197) | | | - | |
| Prepaid expenses, other current assets, and other assets | (2,067) | | | 53 | |
| Accounts payable, accrued expenses and other liabilities | 1,631 | | | 704 | |
| Net cash used in operating activities | (7,205) | | | (5,477) | |
| Cash flows from investing activities: | | | |
| Purchase of marketable debt securities | (15,053) | | | - | |
| Redemption of marketable debt securities | 9,030 | | | - | |
| Net cash used in investing activities | (6,023) | | | - | |
| Cash flows from financing activities: | | | |
| Proceeds from issuance of convertible note | 230 | | | 2,250 | |
| Proceeds from Initial Public Offering and overallotment | 22,425 | | | - | |
| Issuance costs related to Initial Public Offering and overallotment | (2,233) | | | - | |
| Proceeds from exercise of options and warrants | 50 | | | - | |
| Net cash provided by financing activities | 20,472 | | | 2,250 | |
| Net increase (decrease) in cash and cash equivalents | 7,244 | | | (3,227) | |
| Cash and cash equivalents at beginning of period | 1,312 | | | 4,539 | |
| Cash and cash equivalents at end of period | $ | 8,556 | | | $ | 1,312 | |
| | | |
| Supplemental disclosure of non-cash financing activities: | | | |
| Right-of-use lease asset and operating lease liability | $ | 155 | | | $ | - | |
| Convertible notes issued in exchange for services | $ | 13 | | | - | |
| Conversion of convertible notes and accrued interest into common stock | $ | 4,737 | | | $ | - | |
| Warrants issued in relation to issuance of convertible notes | $ | 159 | | | $ | - | |
| Warrants issued to underwriter in connection with stock issuance | $ | 1,170 | | | $ | - | |
| Preferred stock deemed dividend | $ | 1,324 | | | $ | - | |
The accompanying notes are an integral part of these financial statements.
INTENSITY THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
Note 1. Description of Business
Intensity Therapeutics, Inc. (“the Company”) is a biotechnology company whose treatment approach addresses both the regional and systemic nature of a patient’s cancer. The Company’s DfuseRxSM technology platform has identified a lead drug, INT230-6. The Company is based in Connecticut and was incorporated in Delaware in December 2012.
As a result of its initial public offering (the “IPO”) that priced on June 29, 2023, the Company began trading on the Nasdaq Capital Market under the symbol “INTS” on June 30, 2023. The IPO closed on July 5, 2023 at the IPO price of $5.00 per share, at which time the Company issued 3,900,000 shares of our common stock for gross proceeds of $19.5 million. After deducting offering expenses of $2.0 million, the Company received net proceeds received of $17.5 million. On July 7, 2023, the Company sold the full over-allotment shares at the IPO price of $5.00 per share, resulting in the issuance of 585,000 shares of our common stock for gross proceeds of $2.9 million. After deducting offering expenses of $0.2 million, the Company received an additional $2.7 million in net cash proceeds. The Company has begun to use and will continue to use the net proceeds from the IPO to initiate clinical studies, conduct manufacturing suitable for phase 3 studies, submit regulatory filings to the United States Food & Drug Administration (“FDA”) and for general and corporate purposes.
In April 2023, the Company effected a two-for-one reverse stock split (the “Reverse Stock Split”). All owners received one issued and outstanding share of the Company’s common stock in exchange for two outstanding shares of the Company’s common stock. All fractional shares created by the two-for-one exchange were paid in cash. The conversion price of Series A redeemable convertible preferred stock, Series B convertible preferred stock, and Series C convertible preferred stock were adjusted to reflect the Reverse Stock Split by doubling the original conversion price. The Reverse Stock Split has no impact on the par value per share of the Company’s common stock, Series A redeemable convertible preferred stock, Series B convertible preferred stock, and Series C convertible preferred stock, all of which remain at $.0001. All holders of options and warrants had the exercise price doubled and the number of shares issuable upon exercise reduced by half. All current and prior period amounts related to shares, share prices and loss per share, presented in the Company’s financial statements and the accompanying notes have been restated for the Reverse Stock Split. All preferred stock and convertible notes were converted into common stock on the IPO date.
Note 2. Liquidity and Plan of Operation
The accompanying financial statements have been prepared in conformity with generally accepted accounting principles, which contemplate continuation of the Company as a going concern.
The Company is a research and development company and has not generated any revenue from its product candidates. The Company has experienced net losses and negative cash flows from operations each year since its inception. Through December 31, 2023, the Company has an accumulated deficit of $50.5 million. The Company’s operations have been financed primarily through the sale of equity securities and convertible notes. The Company’s net loss for the year ended December 31, 2023 was $10.5 million. The Company expects to incur significant expenses to complete development of its product candidates. The Company may never be able to obtain regulatory approval for the marketing of any of its product candidates in the United States or internationally and there can be no assurance that the Company will generate revenues or ever achieve profitability. The Company does not expect to receive significant product revenue in the near term. The Company, therefore, expects to continue to incur substantial losses for the foreseeable future.
Cash, cash equivalents and marketable debt securities totaled $14.8 million as of December 31, 2023. Until such time the Company can generate substantial product revenue, the Company expects to finance its operations through a combination of equity offerings and convertible debt financings. The Company does not have any committed external source of funds. To the extent that the Company can raise additional capital through the sale of equity or convertible debt securities, the ownership interest of the Company stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of the common stockholders. If the Company is unable to raise additional funds through equity or debt financings when needed, the Company may be required to delay, limit, reduce or terminate its research and product development.
Based on the cash, cash equivalents, and marketable debt securities as of December 31, 2023, the Company believes that it has cash through the end of the first quarter of 2025 for its current operations. As a result, the Company believes there is substantial doubt about its ability to continue as a going concern.
Note 3. Basis of Presentation and Summary of Significant Accounting Policies
Basis of presentation
The accompanying financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America (“GAAP”) and reflect the operations of the Company. The Company neither owns nor controls any subsidiary companies.
Financial impact of events beyond our control
Our financial condition and results of operations may be impacted by factors we may not be able to control, such as the COVID-19 or other pandemic, global supply chain disruptions, global trade disputes and/or political instability. Increases in interest rates, especially if coupled with reduced government spending and volatility in financial markets, may have the effect of further increasing economic uncertainty and heightening these risks. Additionally, rising inflation rates may affect us by increasing operating expenses, such as employee-related costs and clinical trial expenses, negatively impacting our results of operations.
The Company’s financial results for the year ended December 31, 2023 were not significantly impacted by COVID-19 or other factors beyond our control, such as those described above. However, the Company cannot predict the impact of any of these factors on future results or the Company’s ability to raise capital due to a variety of factors, including but not limited to the continued good health of Company employees, the ability of service providers and suppliers to continue to operate and deliver, the ability of the Company to maintain operations, and any government and/or public actions taken in response to these factors.
Use of estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
Certain accounting principles require subjective and complex judgments to be used in the preparation of financial statements. Accordingly, a different financial presentation could result depending on the judgments, estimates, or assumptions that are used.
The Company utilizes significant estimates and assumptions in valuing its stock-based awards and accruals of research and development expenses. An additional significant estimate is that these financial statements are based on the assumption of the Company continuing as a going concern.
Concentration of credit risk
The Company’s financial instruments that are exposed to concentrations of credit risk consist entirely of cash and investments in U.S. Treasury bills. These financial instruments are held at two U.S. financial institutions. The cash accounts are insured by the Federal Deposit Insurance Corporation (“FDIC”) up to regulatory limits. During the years ended December 31, 2023 and 2022, the Company’s cash balances exceeded the FDIC insurance limit. The investments in the U.S. Securities money market fund and U.S. Treasury bills are not FDIC insured but are backed by the U.S. government. U.S. Treasury bills are subject to market risk if they are sold prior to maturity. The Company has not experienced any losses in such accounts. Although the Company believes that the financial institutions with whom the Company does business will be able to fulfill their commitments to the Company, there is no assurance that those institutions will be able to continue to do so beyond amounts guaranteed by the FDIC.
Cash and cash equivalents
The Company considers all liquid investments acquired with a maturity of three months or less to be cash equivalents.
Marketable debt securities
Investments in U.S. Treasury bills purchased with a maturity over three months but less than twelve months are classified separately from cash and cash equivalents in current assets. Investments in U.S. Treasury bills are classified as available for sale. Under the classification of available for sale, securities are reported at fair value. Unrealized gains or losses would be included in accumulated other comprehensive income within the equity section of the Balance Sheet. At December 31, 2023, there were no unrealized gains or losses and all accrued interest was recognized as Interest income in the Statement of Operations.
Fair value measurement
The Company reports its investments at fair value. Fair value is an estimate of the exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants (i.e., the exit price at the measurement date). Fair value measurements are not adjusted for transaction costs. A fair value hierarchy provides for prioritizing inputs to valuation techniques used to measure fair value into three levels:
| | | | | | | | |
| Level 1 | Unadjusted quoted prices in active markets for identical assets or liabilities. |
| | |
| Level 2 | Inputs other than quoted market prices that are observable, either directly or indirectly, and reasonably available. Observable inputs reflect the assumptions market participants would use in pricing the asset or liability and are developed based on market data obtained from sources independent of the Company. |
| | |
| Level 3 | Unobservable inputs. Unobservable inputs reflect the assumptions that the Company develops based on available information about what market participants would use in valuing the asset or liability. |
An asset’s or liability’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. Availability of observable inputs can vary and is affected by a variety of factors. The Company uses judgment in determining fair value of assets and liabilities and Level 3 assets and liabilities involve greater judgment than Level 1 or Level 2 assets or liabilities.
As of December 31, 2023, the Company invested $6.2 million in U.S. Treasury Bills, included in marketable debt securities. U.S. Treasury bills are valued at market prices obtained from independent vendor services, which we believe to be reliable. In some cases, the pricing vendor may provide prices quoted by a single broker or market maker. U.S. Treasury Bills are categorized in Level 2 of the fair value hierarchy. As of December 31, 2022, there were no investments in U.S. Treasury Bills.
The Company’s financial instruments, including cash equivalents and current liabilities are carried at cost, which approximates fair value due to the short-term nature of these instruments.
Stock-based compensation
The Company accounts for stock-based compensation to employees and non-employees, which consists of stock option grants, through the Statements of Operations based on their fair values at the date of grant.
The Company calculates the fair value of option grants utilizing the Black-Scholes pricing model. The resulting stock-based compensation expense for both employee and non-employee awards is generally recognized on a straight-line basis over the requisite service period of the award. Forfeitures are recognized as they occur.
The Company had been a private company and lacked company-specific historical and implied volatility information for its shares. Therefore, the Company estimated its expected share price volatility based on the historical volatility of publicly traded peer companies and expects to continue to do so until such time as it has adequate historical data regarding the volatility of its own traded share price.
Research and development and patent costs
The Company is required to estimate its expenses resulting from its obligations under contracts with vendors, consultants, contract research organizations (“CRO”), and contract manufacturing organizations (“CMO”) in connection with conducting research and development activities. The financial terms of these contracts vary from contract to contract and may result in payment flows that do not match the periods over which materials or services are provided under such contracts.
Research and development costs are expensed in the period in which they are incurred. External costs consist primarily of payments to outside consultants, third-party CROs, CMOs, clinical trial sites and central laboratories in connection with the Company’s clinical manufacturing and clinical development activities. External expenses are recognized based on an evaluation of the progress to completion of specific tasks using information provided to the Company by its service providers or its estimate of the level of service that has been performed at each reporting date. The Company tracks external costs based on research and development initiative, including preclinical, individual clinical study, and manufacturing activities. Internal costs consist primarily of employee-related costs and costs related to compliance with regulatory requirements. The Company does not track internal costs by program because these costs are deployed across multiple programs and, as such, are not separately classified.
The Company makes estimates of accrued expenses as of each balance sheet date based on facts and circumstances known at that time. The Company periodically confirms the accuracy of its estimates with the service providers and makes adjustments if necessary. The significant estimates in its accrued research and development expenses include the costs incurred for services performed by vendors in connection with research and development activities for which the Company has not yet been invoiced.
In mid-2024, the Company intends on initiating a Phase 3 open-label, randomized study for certain soft tissue sarcoma subtypes. In connection with this study, the Company recorded an advance payment of $1.7 million, which will be applied to invoices at the end of the study and is included in Other Assets in the Balance Sheet as of December 31, 2023, as the Phase 3 study is expected to span several years.
Income taxes
The Company accounts for income taxes through the use of the asset-and-liability method whereby deferred tax assets and liabilities are determined based on differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company utilizes a valuation allowance to reduce deferred tax assets to their estimated realizable value.
The Company accounts for uncertain tax positions. When uncertain tax positions exist, the Company recognizes the tax benefit of tax positions to the extent that the benefit will more likely than not be realized. The determination as to whether the tax benefit will more likely than not be realized is based upon the technical merits of the tax position as well as consideration of the available facts and circumstances. As of December 31, 2023, the Company does not have any significant uncertain tax positions.
There are no estimated interest costs and penalties provided for in the Company’s financial statements for the year ended December 31, 2023. If at any time the Company should record interest and penalties in connection with income taxes, the interest and penalties will be expensed within the income tax line.
Leases
The Company determines if an arrangement contains a lease at contract inception. With the exception of short-term leases (leases with terms less than 12 months), all leases with contractual fixed costs are recorded on the balance sheet on the commencement date as a right-of-use (ROU) asset and a lease liability. Lease liabilities to be paid over the next twelve months are classified as current lease liability and all other lease obligations are classified as long-term lease liability. Lease liabilities are initially measured at the present value of the future minimum lease payments and subsequently increased to reflect the interest accrued and reduced by the lease payments made. The Company’s building leases require a pro-rata share of operating expenses and real estate taxes, which are variable in nature and excluded from the measurement of lease liabilities. ROU assets are initially measured at the present value of the future minimum lease payments adjusted for any prior lease prepayments, lease incentives and initial direct costs. Certain leases contain escalation, renewal and/or termination options that are factored into the ROU asset as appropriate. Operating leases result in a straight-line rent expense over the expected lease term.
The Company uses its estimated incremental borrowing rate, which is derived from information available at the lease commencement date, in determining the present value of future lease payments, if the rate implicit in the lease is not readily determinable. Consideration is given to publicly available data for instruments with similar characteristics when calculating incremental borrowing rates. This incremental borrowing rate estimate is based on a synthetic credit rating derived from the market capitalization of similar companies, the treasury yield curve, and corporate yield spreads.
Basic and dilutive loss per share
Basic net loss per share is determined using the weighted average number of shares of common stock outstanding during each period. Dilutive net loss per share includes the effect, if any, from the potential exercise or conversion of securities, such as convertible preferred stock, convertible notes, stock options, and stock warrants, which would result in the issuance of incremental shares of common stock. The computation of diluted net loss per share does not include the conversion of securities that would have an anti-dilutive effect. Potential shares of common stock issuable upon conversion of preferred stock, exercise of stock options, and exercise of warrants that are excluded from the computation of diluted weighted average shares outstanding listed in the table below because they are anti-dilutive. The basic and diluted computation of net loss per share for the Company are the same because the effects of the Company’s convertible securities would be anti-dilutive. All common and preferred stock participate equally in dividends and the distribution of earnings if and when declared by the Board of Directors, on the Company’s common stock for the year ended December 31, 2023. For purposes of computing earnings per share, all series of preferred stock are considered participating securities. Therefore, the Company must calculate basic and diluted earnings per share using the two-class method. Under the two-class method, net income for the period is allocated between common stockholders and participating securities according to dividends declared and participation rights in undistributed earnings. As the preferred stockholders have no obligation to fund losses, no portion of net loss was allocated to the participating securities for the year ended December 31, 2022. There were no preferred shares outstanding at December 31, 2023.
As of December 31, 2023 and 2022, the following shares of common stock underlying preferred stock, options, and warrants were excluded from the computation of diluted weighted average shares outstanding. In accordance with the Reverse Stock Split in April 2023 (see Note 1), the number of shares of common stock underlying the preferred stock, options and warrants are now half, and the below information gives effect to this Reverse Stock Split:
| | | | | | | | | | | |
| December 31, |
| 2023 | | 2022 |
| Preferred stock Series A outstanding | - | | 2,499,999 |
| Preferred stock Series B outstanding | - | | 724,552 |
| Preferred stock Series C outstanding | - | | 900,300 |
| Options outstanding | 1,239,750 | | 1,044,250 |
| Warrants outstanding | 801,950 | | 357,750 |
| 2,041,700 | | 5,526,851 |
As of December 31, 2022, the shares that would be issued from the convertible notes outstanding are also excluded from diluted weighted average shares outstanding, since the conversion rate is dependent upon qualified liquidity events. All convertible notes were converted into shares of common stock on June 29, 2023.
Stock issuance costs
The Company incurred costs related to the sale of its common stock in its IPO and the subsequent sale of common stock in the over-allotment. These costs included underwriter commissions and fees, legal fees, accounting fees, and printing costs. These costs were recorded as a deduction to Additional Paid in Capital.
Recently issued pronouncements
The Company does not believe that any recently issued, but not yet effective, accounting pronouncements, if currently adopted, would have a material impact on its financial statements.
Reclassifications
Certain prior year amounts have been reclassified to conform to current year presentation.
Note 4. Cash and Cash Equivalents
Cash and cash equivalents consisted of the following (in thousands):
| | | | | | | | | | | |
| Savings and checking accounts at major U.S. financial institutions | $ | 367 | | | $ | 1,312 | |
| U.S. Treasury securities money market fund | 8,189 | | | - | |
| | | |
| Total | $ | 8,556 | | | $ | 1,312 | |
Note 5. Marketable Debt Securities
Marketable debt securities as of December 31, 2023 consisted entirely of U.S. Treasury bills purchased with maturities over three months but less than twelve months. There were no marketable debt securities as of December 31, 2022.
Note 6. Prepaid Expenses
Prepaid expenses consisted of the following (in thousands):
| | | | | | | | | | | |
| December 31, |
| 2023 | | 2022 |
| Prepaid insurance | $ | 647 | | | $ | 29 | |
| Prepaid other | 35 | | | 34 | |
| Total | $ | 682 | | | $ | 63 | |
Note 7. Accrued Expenses
Accrued expenses consisted of the following (in thousands):
| | | | | | | | | | | |
| December 31, |
| 2023 | | 2022 |
| Clinical study patient costs incurred but not yet invoiced | $ | 439 | | | $ | 1,393 | |
| Accrued vacation, wages, bonuses, and related payroll taxes | 392 | | | 329 | |
| Accrued other | 60 | | | 2 | |
| Total | $ | 891 | | | $ | 1,724 | |
Note 8. Convertible Notes
Prior to the IPO, the Company entered into a series of interest-bearing convertible notes as described below.
In September 2021, the Company entered into a convertible note agreement with a shareholder for aggregate principal of $2.0 million, as amended in November 2022 (the “2021 Convertible Note”). The outstanding principal balance and accrued interest on the note automatically converted upon the IPO at a discount of 35% to the conversion price of $11.50 per share in accordance with the note agreement. The 2021 Convertible Note had accrued interest at 3% per annum.
In November 2022, the Company entered into convertible note agreements with shareholders for an aggregate $2.3 million with three holders (the “2022 Convertible Notes”). The outstanding principal balance and accrued interest on these notes automatically converted upon the IPO at a discount of 30% to the conversion price of $11.50 per share in accordance with the note agreement. The 2022 Convertible Notes had accrued interest at 10% per annum.
In March through May 2023, the Company entered into convertible note agreements for an aggregate $0.2 million with four holders (the “2023 Convertible Notes”). The outstanding principal balances and accrued interest on these notes
automatically converted upon the IPO at a discount of 30% to the conversion price of $11.50 per share in accordance with the note agreement. The 2023 Convertible Notes had accrued interest at 10% per annum.
Convertible notes consisted of the following as of December 31, 2023 and 2022 (in thousands):
| | | | | | | | | | | | | | | | | |
| Principal | | Accrued Interest | | Total |
| As of January 1, 2022 | $ | 2,000 | | | $ | 17 | | | $ | 2,017 | |
| Issuance of 2022 Convertible Notes | 2,250 | | | — | | | 2,250 | |
| Interest expense - 2022 | — | | | 82 | | | 82 | |
| Balance as of December 31, 2022 | 4,250 | | | 99 | | | 4,349 | |
| Issuance of 2023 Convertible Notes | 243 | | | — | | | 243 | |
| Interest expense - 2023 | — | | | 145 | | | 145 | |
| Conversion to common stock upon IPO | (4,493) | | | (244) | | | (4,737) | |
| Balance as of December 31, 2023 | $ | — | | | $ | — | | | $ | — | |
As of December 31, 2022, the Company classified the convertible notes as a current liability since the Company anticipated that these notes would automatically convert into shares of common stock within one year.
Upon the IPO, all convertible note principal and accrued interest of $4.5 million and $0.2 million, respectively, converted into an aggregate of 1,399,716 shares of common stock, pursuant to the conversion terms in each respective note. The Company recorded a non-operating loss on debt extinguishment of $2.3 million in the statement of operations, which is equal to the aggregate discounts on the IPO price specified in each convertible note agreement. In addition, upon the IPO, the remaining $0.2 million balance of unamortized discount on 2022 Convertible Notes was recognized and recorded in Interest Expense on the Statement of Operations.
Note 9. Stockholders’ Equity
Initial public offering
On June 29, 2023, as described in Note 1, the Company priced its IPO, issuing 3,900,000 shares of common stock at the IPO price of $5.00 per share. On July 7, 2023, the Company sold the full over-allotment of the IPO shares and issued 585,000 shares of common stock at the IPO price of $5.00 per share.
On June 29, 2023, pursuant to the IPO,
•all Series A, B and C Preferred Stock was converted into 4,124,851 shares of common stock at conversion prices equal to $4.00, $9.00 and $11.50, respectively..
•Series B and Series C Preferred Stock received an additional 100,189 and 164,518 shares of common stock, respectively, pursuant to the terms of their security agreements, since the IPO price was less than the original issue price. The additional shares have been recorded as a deemed dividend on the Statement of Operations and Statement of Changes in Redeemable Convertible Preferred Stock and Stockholders’ Equity (Deficiency).
•All voting, dividend, redemption and liquidation preference rights, specific to the Series A, B, and C Preferred Stock were extinguished.
•All Convertible Notes and related accrued interest were converted into 1,399,716 shares of common stock, as described in Note 8.
Authorized shares
Pursuant to the sixth amended and restated Certificate of Incorporation, dated June 30, 2023, the total number of shares of all classes of stock which the Company shall have authority to issue is (i) 135,000,000 shares of common stock and (ii) 15,000,000 shares of preferred stock.
Note 10. Stock Based Compensation
The Company has a 2013 Stock Option Plan (the “2013 Plan”), which is administered by our Compensation Committee. Under the 2013 Plan, stock options to purchase shares of common stock could be granted to eligible employees, officers, directors and consultants of the Company.
In 2021, the Company replaced the 2013 Plan with the 2021 Stock Incentive Plan (the “2021 Plan”), authorizing the granting of equity awards for the issuance of up to 3,000,000 shares of common stock. Upon adoption of the 2021 Plan, no more shares would be issued under the 2013 Plan. Starting on January 1, 2022, the shares authorized under the 2021 Plan shall have an annual increase of the lessor of (a) 3.5% of the aggregate number of shares of Common Stock outstanding on the final day of the preceding calendar year, or (b) such smaller amount as determined by the Board. On January 1, 2023, an additional 238,700 shares were authorized under the 2021 Plan. As of December 31, 2023, 2,837,700 shares were available for issuance under the 2021 Plan.
The Company recorded total stock-based compensation in its Statements of Operations as follows (in thousands):
| | | | | | | | | | | | | | | |
| | | Years Ended December 31, |
| | | | | 2023 | | 2022 |
| Research and development | | | | | $ | 714 | | | $ | 804 | |
| General and administrative | | | | | 684 | | | 365 | |
| Total stock-based compensation expense | | | | | $ | 1,398 | | | $ | 1,169 | |
Stock options
The following table summarizes the range of assumptions used to estimate the fair value of stock options issued in 2023 and 2022:
| | | | | | | | | | | |
| 2023 | | 2022 |
| Stock price | $2.82 to 6.88 | | $4.50 |
| Exercise price | $2.82 to 6.88 | | $4.50 |
| Expected volatility | 97.06 to 99.74% | | 103.85% |
| Risk free interest rates | 3.87 to 4.97% | | 3.59% |
| Expected term (years) | 5.75 to 6.25 | | 3 to 4 |
For the years ended December 31, 2023 and 2022, a dividend yield of 0% was used because the Company has not historically paid and does not intend to pay a dividend on Common Stock in the foreseeable future. The expected stock price volatility assumption was estimated based on the historical volatilities for industry peers, as the Company had no active market for its stock prior to the IPO and limited history for issuance price of its stock. The risk-free rate assumption is determined using the yield currently available on U.S. Treasury zero coupon issues with a remaining term commensurate with the expected term of the award. The expected term of the option represents the period the options are expected to be outstanding.
The following table summarizes the activity for stock options for the year ended December 31, 2023:
| | | | | | | | | | | | | | | | | | | | | | | |
| Options | | Weighted-
Average
Exercise Price | | Weighted Average
Remaining
Contractual Term
(in years) | | Aggregate
Intrinsic
Value (in
thousands) |
| Outstanding at December 31, 2022 | 1,044,250 | | $ | 8.48 | | | 6.6 | | $ | 235 | |
| Issued | 208,000 | | $ | 5.25 | | | | | |
| Exercised | (12,500) | | $ | 2.00 | | | | | |
| Forfeited and cancelled | - | | $ | — | | | | | |
| Outstanding at December 31, 2023 | 1,239,750 | | $ | 8.00 | | | 6.4 | | $ | 1,865 | |
| Exercisable at December 31, 2023 | 850,250 | | $ | 8.15 | | | 5.2 | | $ | 1,201 | |
All options expire 10 years from date of grant. Options outstanding begin to expire in August 2024. Options that were granted to employees and consultants have vesting periods that vary by award to recipient and range from immediate vesting to a period of up to 4 years.
The weighted average grant date fair value of stock options issued was $4.21 and $2.96 for the years ended December 31, 2023 and 2022, respectively.
As of December 31, 2023, total unrecognized compensation cost related to options was approximately $1,848,000 and is expected to be recognized over the remaining weighted average service period of 2.2 years.
Warrants
The following table summarizes the range of assumptions used to estimate the fair value of warrants issued in 2023 and 2022:
| | | | | | | | | | | |
| 2023 | | 2022 |
| Stock price | $3.10 to $5.00 | | $4.50 |
| Exercise price | $6.00 to $6.25 | | $4.50 |
| Expected volatility | 97.06% to 103.85% | | 103.85% |
| Risk free interest rates | 3.59% to 4.85% | | 3.59% |
| Expected term (years) | 3 to 5 | | 10 |
For the years ended December 31, 2023 and 2022, a dividend yield of 0% was used because the Company has not historically paid and does not intend to pay a dividend on Common Stock in the foreseeable future. The expected stock price volatility assumption was estimated based on the historical volatilities for industry peers, as the Company had no active market for its stock prior to the IPO and limited history for issuance price of its stock. The risk-free rate assumption is determined using the yield currently available on U.S. Treasury zero coupon issues with a remaining term commensurate with the expected term of the award. The expected term of the option represents the period the options are expected to be outstanding.
The following table summarizes the activity for warrants for the year ended December 31, 2023:
| | | | | | | | | | | | | | | | | | | | | | | |
| Warrants | | Weighted-
Average
Exercise Price | | Weighted Average
Remaining Contractual Term
(in years) | | Aggregate
Intrinsic
Value (in
thousands) |
| Outstanding at December 31, 2022 | 357,750 | | $ | 6.00 | | | 4.8 | | $ | 183 | |
| Issued | 459,950 | | $ | 6.46 | | | | | |
| Exercised | (12,500) | | $ | 2.00 | | | | | |
| Forfeited and cancelled | (3,250) | | $ | 11.50 | | | | | |
| Outstanding at December 31, 2023 | 801,950 | | $ | 6.30 | | | 3.9 | | $ | 2,096 | |
| Exercisable at December 31, 2023 | 699,200 | | $ | 6.26 | | | 3.8 | | $ | 1,876 | |
All warrants outstanding are exercisable for purchase of common stock. In connection with the IPO, 313,950 warrants were issued to the Company’s underwriters during the year ended December 31, 2023.
At December 31, 2023, total unrecognized compensation cost related to warrants was approximately $282,000 and is expected to be recognized over the remaining weighted average service period of 1.4 years.
Note 11. Leases
In January 2017, the Company entered into a lease for approximately 2,500 square feet of office space in Westport, Connecticut, (the “Westport Lease”), which was subsequently extended and increased to approximately 4,000 square feet. In June 2023, the Westport Lease was terminated.
In July 2023, the Company signed a 5.5-year lease for approximately 2,700 square feet of office space in Shelton, Connecticut, (the “Shelton Lease”). The Company has a one-time option to cancel the Shelton Lease after 36 months if it provides written notice before the end of month 30. A payment of approximately $47,000 would be due at the end of month 36 if the Company exercises this option. This option is not reasonably certain to occur.
Rent expense for the years ended December 31, 2023 and 2022 was $63,000 and $120,000, respectively. Cash paid for operating leases for the years ended December 31, 2023 and 2022 was approximately $49,000 and $192,000, respectively, all of which pertained to the Shelton Lease. Cash paid for the Westport Lease will commence in 2024.
The following table summarizes the balance sheet classification of the operating lease asset and related lease liabilities as of December 31, 2023 for the Shelton Lease and as of December 31, 2022 for the Westport Lease (in thousands):
| | | | | | | | | | | |
| December 31,
2023 | | December 31,
2022 |
| | | |
| Right-of-use asset, net | $ | 147 | | | $ | 139 | |
| | | |
| Lease liability, current portion | 20 | | | 143 | |
| Lease liability, net of current portion | 138 | | | — | |
| $ | 158 | | | $ | 143 | |
The following variables were used to determine the right-of-use asset and the operating lease liabilities at December 31, 2022 and 2021:
| | | | | | | | | | | |
| December 31,
2023 | | December 31,
2022 |
| Weighted average remaining lease term | 5.2 years | | 0.75 years |
| Weighted average operating lease discount rate | 6.4 | % | | 3.9 | % |
Future minimum lease payments under the lease agreement as of December 31, 2023 were as follows (in thousands):
| | | | | |
| Year ended | |
| 2024 | $ | 29 | |
| 2025 | 36 | |
| 2026 | 37 | |
| 2027 | 39 | |
| 2028 and thereafter | 46 | |
| Total lease payments | $ | 187 | |
| Less: Amounts representing interest | (29) | |
| Present value of lease liabilities | $ | 158 | |
Note 12. Other Uncertainties
The Company holds one of its patents in Russia. The payment for this patent is paid until September 15, 2024. If subsequent payments to Russia are restricted, the Company may lose this patent in Russia. The Company has no other significant business activities in Belarus, Russia or the Ukraine. The Company also holds a patent in Israel which is currently involved in military action.
Note 13. Related Parties
Beginning in February 2022, a minority stockholder was engaged as a consultant to serve as the Company’s chief medical officer. Under the consulting agreement, the Company recorded approximately $6,200 and $96,000 of research and development expense for the years ended December 31, 2023 and 2022, respectively.
The Company sublet a portion of its Westport Lease to a minority stockholder, and recognized sublease income of approximately $23,000 and $68,000 for the years ended December 31, 2023 and 2022. Sublease income is recorded as a reduction of general and administrative expenses in the Statement of Operations. The sublease ended in April 2023.
As of December 31, 2023 and 2022, the Company held a $36,000 deposit related to a service agreement with a minority stockholder, and is recorded in other long-term liabilities on the balance sheet. The deposit will be returned to the minority stockholder at the completion of the service agreement.
In October 2023, the Company issued 80,000 warrants for consulting services to be rendered by the two shareholders, which will vest over the subsequent twelve months. These warrants are valued at $198,000 and will be expensed to general and administrative expense over the subsequent twelve month period.
Note 14. Income Taxes
The components of the Company’s provision for income taxes and income taxes computed using the U.S. federal statutory corporate tax rate were as follows (in thousands):
| | | | | | | | | | | |
| 2023 | | 2022 |
| | | |
| Statutory federal income tax rate | $ | (2,213) | | | $ | (1,592) | |
| State taxes, net of federal tax benefit | (350) | | | (131) | |
| Change in valuation allowance | 1,888 | | | 1,543 | |
| Loss on debt extinguishment | 475 | | | — | |
| Federal return to provision | 87 | | | — | |
| Other permanent items | 113 | | | 180 | |
| Provision for income taxes | $ | — | | | $ | — | |
The components of the net deferred tax assets are as follows (in thousands):
| | | | | | | | | | | |
| 2023 | | 2022 |
| | | |
| Deferred tax assets: | | | |
| Net operating loss carryforwards | $ | 8,612 | | | $ | 8,055 | |
| Capitalized research expense | 1,782 | | | 823 | |
| Share based compensation | 1,011 | | | 602 | |
| Research and development credits | 596 | | | 636 | |
| Lease liability | 43 | | | 30 |
| Gross deferred tax assets | 12,044 | | | 10,146 | |
| Less valuation allowance | (12,005) | | | (10,116) | |
| Total deferred tax assets | 39 | | | 30 | |
| | | |
| Deferred tax liabilities: | | | |
| Right of use assets | (39) | | | (30) |
| Total deferred tax liabilities | (39) | | | (30) | |
| Deferred income taxes, net | $ | — | | | $ | — | |
As of December 31, 2023, the Company has U.S. federal and state net operating loss carryforwards of $32.0 million and $31.9 million, respectively, which may be used to offset future taxable income, if any. As of December 31, 2022, the Company had U.S. federal and state net operating loss carryforwards of $28.1 million and $28.0 million, respectively, which may be used to offset future taxable income, if any. The Company’s U.S. federal and state net operating loss carryforwards begin to expire in 2033 and the U.S. federal net operating losses generated between 2018 and 2022 can be carried forward indefinitely. Federal loss carryforwards of $7.0 million expire between the years 2033 and 2037, and the remainder have no expiration date.
As of December 31, 2023 and 2022, the Company has U.S. federal and state credit carryforwards of $0.4 million and $0.2 million, respectively, which may be used to offset future taxable income, if any. The Company’s U.S. federal and state credit carryforwards begin to expire in 2033. The Company’s ability to utilize these net operating loss carryforwards and tax credit carryforwards may be limited if the Company experiences an ownership change pursuant to Internal Revenue Code Section 382 and 383. An ownership change occurs when the ownership percentages of 5% or greater stockholders change by more than 50% over a three-year period. The Company has not completed an analysis under Section 382 of the Code.
A valuation allowance for deferred tax assets is recorded when it is more likely than not that some or all of the benefit from the deferred tax asset will not be realized. The Company provides a valuation allowance to offset deferred tax assets for net operating losses incurred during the year and for other deferred tax assets where, in the Company’s opinion, it is more likely than not that the financial statement benefit of these losses will not be realized.
The Company’s policy is to classify interest and penalties, if any, as components of the income tax provision in the statement of operations. The Company has not recorded any unrecognized tax benefit, interest or penalty in the years ended December 31, 2023 and 2022.
The Company is subject to income tax in the U.S. Federal and Connecticut jurisdictions.