
A New Weapon in the War on Cancer P R E S E N TAT I O N April 2025 Issuer free writing Prospectus Filed Pursuant to Rule 433 Registration No. 333-286683 April 22, 2025

3 3 This presentation highlights basic information about Intensity Therapeutics, Inc. (the “Company”) and the proposed offering. Because it is a summary that has been prepared solely for informational purposes, it does not contain all of the information that you should consider before investing in our company. Except as otherwise indicated, this presentation speaks only as of the date hereof. This presentation does not constitute an offer to sell, nor a solicitation of an offer to buy, these securities in any state or jurisdiction in which such offer solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction by any person in any jurisdiction in which it is unlawful for such person to make such an offering or solicitation. Neither the Securities and Exchange Commission (“SEC”) nor any other regulatory body has approved or disapproved of the securities or passed upon the accuracy or adequacy of this presentation. Any representation to the contrary is a criminal offense. This presentation includes industry and market data that we obtained from industry publications and journals, third party studies and surveys, internal company studies and surveys and other publicly available information. Industry publications and surveys generally state that the information contained therein has been obtained from sources believed to be reliable. Although we believe the industry and market data to be reliable as of the date of this presentation, this information could prove to be inaccurate. Although we believe the industry and market data to be reliable as of the date of this presentation, this information could prove to be inaccurate. Industry and market data could be wrong because of the method by which sources obtained their data and because information cannot always be verified with complete certainty due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties. In addition, we do not know all of the assumptions that were used in preparing the forecasts from the sources relied upon or cited herein. A registration statement on Form S-1 (file number 333-[ ]), including a preliminary prospectus, relating to the offering of securities has been filed by the Company with the SEC. The registration statement has not yet become effective. Before you invest, you should read the preliminary prospectus in the registration statement and, when available, the final prospectus relating to the offering. An electronic copy of the preliminary prospectus relating to the offering is available, and a copy of the final prospectus relating to the offer will be available, on the website of the SEC at www.sec.gov. Copies of the preliminary prospectus and the final prospectus relating to the offering, when available, may be obtained by contacting A.G.P./Alliance Global Partners at prospectus@allianceg.com and Brookline Capital Markets, a division of Arcadia Securities, LLC at michael.fontaine@brooklinecapmkts.com Disclosure Free Writing Prospectus

4 4 This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties, including statements regarding the development and regulatory status of our product candidates, such as statements with respect to our lead product candidate INT230-6, and the timing of clinical trials and data from those trials for our product candidates, and our discovery programs that may lead to our development of additional product candidates, the potential utility of our technology and therapeutic potential of our product candidates, the potential commercialization of any of our product candidates, and the sufficiency of our cash resources. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make as a result of various risks and uncertainties, including but not limited to: the initiation, timing, progress and results of future preclinical studies and clinical trials, and our research and development programs; our need to raise additional funding before we can expect to generate any revenues from product sales; our plans to develop and commercialize our product candidates, and other factors included in the “Risk Factors” section of the Company’s filings with the SEC in the future. Any of these outcomes could cause our actual results to differ from those contained in the forward-looking statements of the Company’s filings with the SEC. The forward-looking statements contained in this presentation reflect our current views as of the date of this presentation with respect to future events, and we assume no obligation to update any forward-looking statements except as required by applicable law. The Intensity Therapeutics, Inc. name and logo are our trademarks. We also own the service mark and the registered U.S. trademark for DfuseRx. The trademarks, trade names and service marks appearing in this presentation are the property of the Company. We have omitted the ® and designations, as applicable, for the trademarks named in this presentation. Forward-Looking Statements
5 • Novel solid tumor cancer treatment approach using a new delivery technology that causes cancer cell death, leading to an immune response • Over 200 patients enrolled in 2 completed clinical trials (1 metastatic & 1 presurgical) • Ongoing studies: Phase 3 in metastatic sarcoma; Phase 2 in presurgical breast cancer • Veteran leadership with public company and phase 3 clinical development experience • Robust IP portfolio – 18 issued patents (3 in the US) and patent protection in 41 countries • Multiple industry, government and university hospital partnerships • Cost-efficient business model structured to create significant value Company Highlights
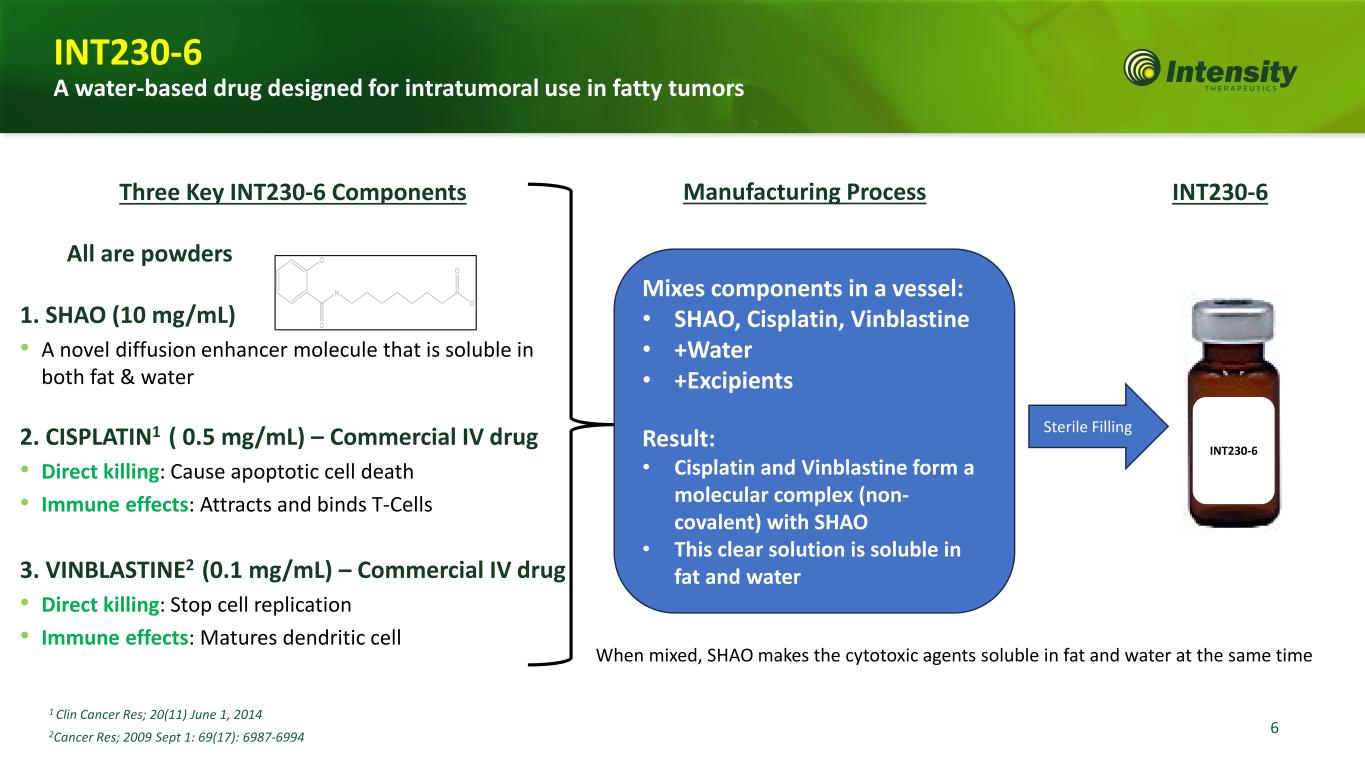
6 Three Key INT230-6 Components All are powders 1. SHAO (10 mg/mL) • A novel diffusion enhancer molecule that is soluble in both fat & water 2. CISPLATIN1 ( 0.5 mg/mL) – Commercial IV drug • Direct killing: Cause apoptotic cell death • Immune effects: Attracts and binds T-Cells 3. VINBLASTINE2 (0.1 mg/mL) – Commercial IV drug • Direct killing: Stop cell replication • Immune effects: Matures dendritic cell 1 Clin Cancer Res; 20(11) June 1, 2014 2Cancer Res; 2009 Sept 1: 69(17): 6987-6994 Mixes components in a vessel: • SHAO, Cisplatin, Vinblastine • +Water • +Excipients Result: • Cisplatin and Vinblastine form a molecular complex (non- covalent) with SHAO • This clear solution is soluble in fat and water INT230-6Manufacturing Process Sterile Filling INT230-6 When mixed, SHAO makes the cytotoxic agents soluble in fat and water at the same time INT230-6 A water-based drug designed for intratumoral use in fatty tumors
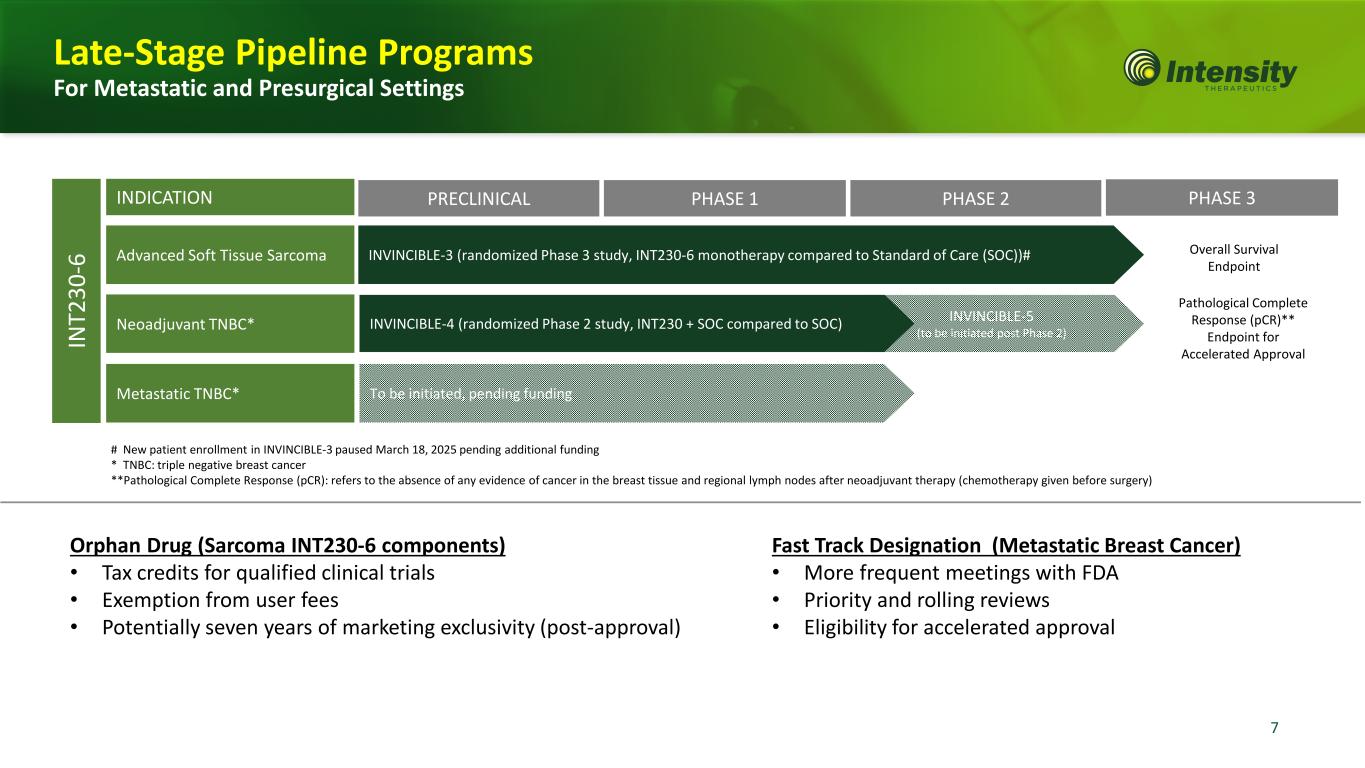
7 Orphan Drug (Sarcoma INT230-6 components) • Tax credits for qualified clinical trials • Exemption from user fees • Potentially seven years of marketing exclusivity (post-approval) To be initiated, pending funding INVINCIBLE-5 (to be initiated post Phase 2) INVINCIBLE-4 (randomized Phase 2 study, INT230 + SOC compared to SOC) INVINCIBLE-3 (randomized Phase 3 study, INT230-6 monotherapy compared to Standard of Care (SOC))# INDICATION IN T2 30 -6 # New patient enrollment in INVINCIBLE-3 paused March 18, 2025 pending additional funding * TNBC: triple negative breast cancer **Pathological Complete Response (pCR): refers to the absence of any evidence of cancer in the breast tissue and regional lymph nodes after neoadjuvant therapy (chemotherapy given before surgery) Fast Track Designation (Metastatic Breast Cancer) • More frequent meetings with FDA • Priority and rolling reviews • Eligibility for accelerated approval Late-Stage Pipeline Programs For Metastatic and Presurgical Settings PRECLINICAL PHASE 1 PHASE 2 PHASE 3 Advanced Soft Tissue Sarcoma Neoadjuvant TNBC* Metastatic TNBC* Overall Survival Endpoint Pathological Complete Response (pCR)** Endpoint for Accelerated Approval
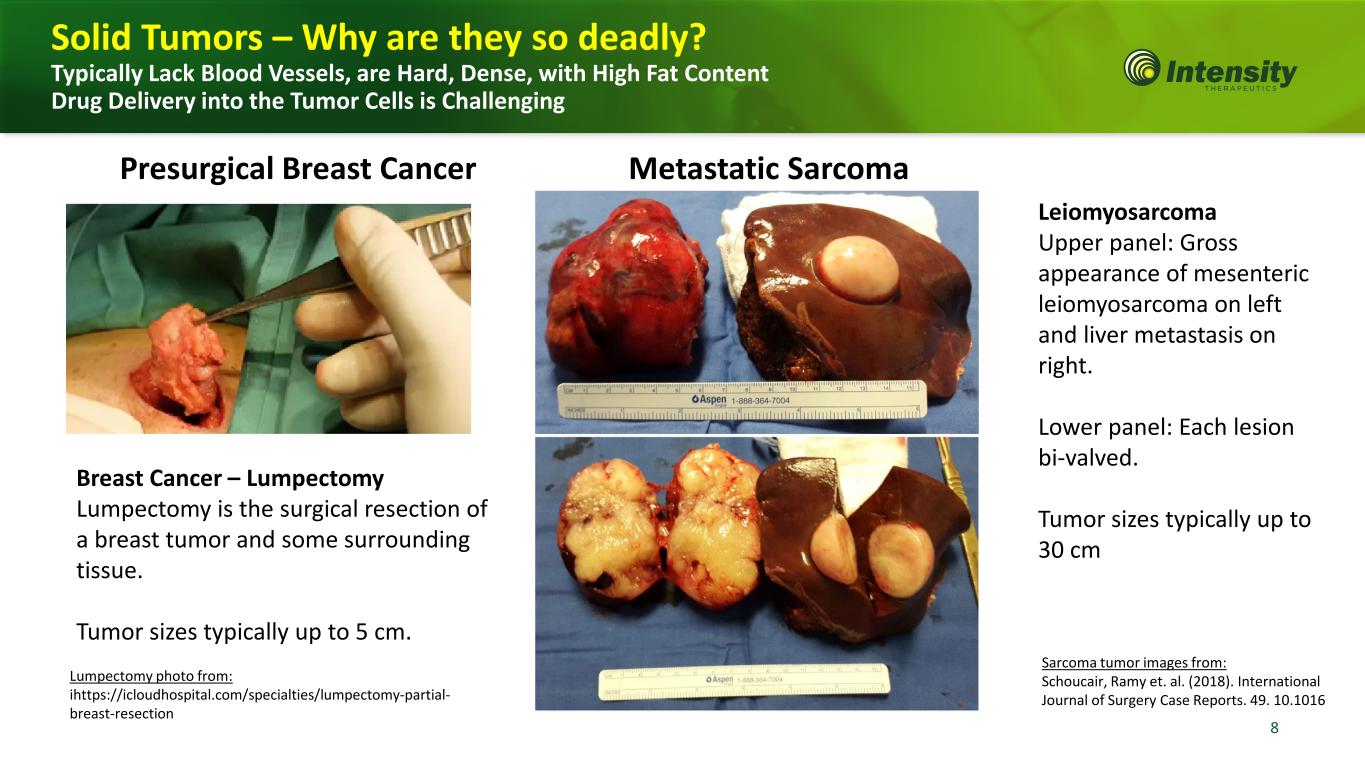
8 8 Leiomyosarcoma Upper panel: Gross appearance of mesenteric leiomyosarcoma on left and liver metastasis on right. Lower panel: Each lesion bi-valved. Tumor sizes typically up to 30 cm Breast Cancer – Lumpectomy Lumpectomy is the surgical resection of a breast tumor and some surrounding tissue. Tumor sizes typically up to 5 cm. Presurgical Breast Cancer Metastatic Sarcoma Solid Tumors – Why are they so deadly? Typically Lack Blood Vessels, are Hard, Dense, with High Fat Content Drug Delivery into the Tumor Cells is Challenging Sarcoma tumor images from: Schoucair, Ramy et. al. (2018). International Journal of Surgery Case Reports. 49. 10.1016 Lumpectomy photo from: ihttps://icloudhospital.com/specialties/lumpectomy-partial- breast-resection
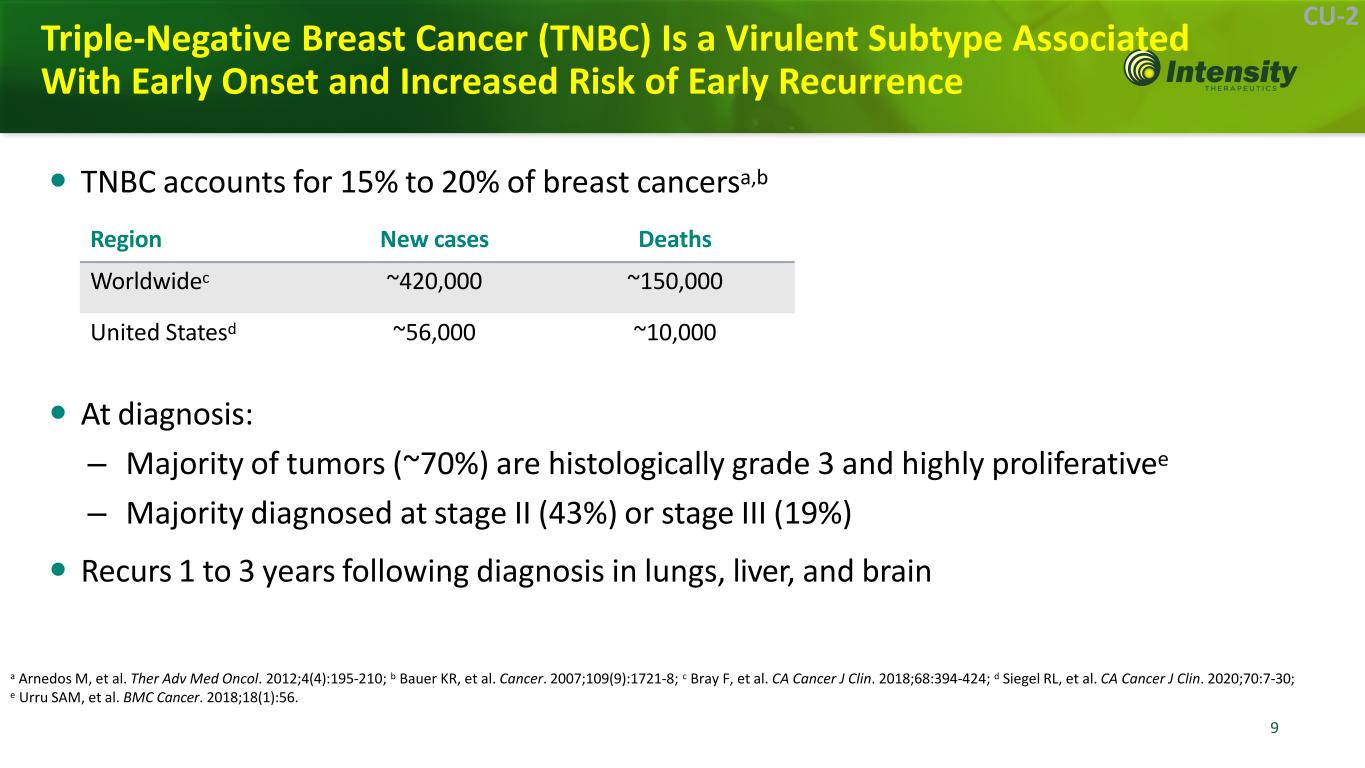
9 9 CU-2 Triple-Negative Breast Cancer (TNBC) Is a Virulent Subtype Associated With Early Onset and Increased Risk of Early Recurrence TNBC accounts for 15% to 20% of breast cancersa,b At diagnosis: – Majority of tumors (~70%) are histologically grade 3 and highly proliferativee – Majority diagnosed at stage II (43%) or stage III (19%) Recurs 1 to 3 years following diagnosis in lungs, liver, and brain a Arnedos M, et al. Ther Adv Med Oncol. 2012;4(4):195-210; b Bauer KR, et al. Cancer. 2007;109(9):1721-8; c Bray F, et al. CA Cancer J Clin. 2018;68:394-424; d Siegel RL, et al. CA Cancer J Clin. 2020;70:7-30; e Urru SAM, et al. BMC Cancer. 2018;18(1):56. Region New cases Deaths Worldwidec ~420,000 ~150,000 United Statesd ~56,000 ~10,000
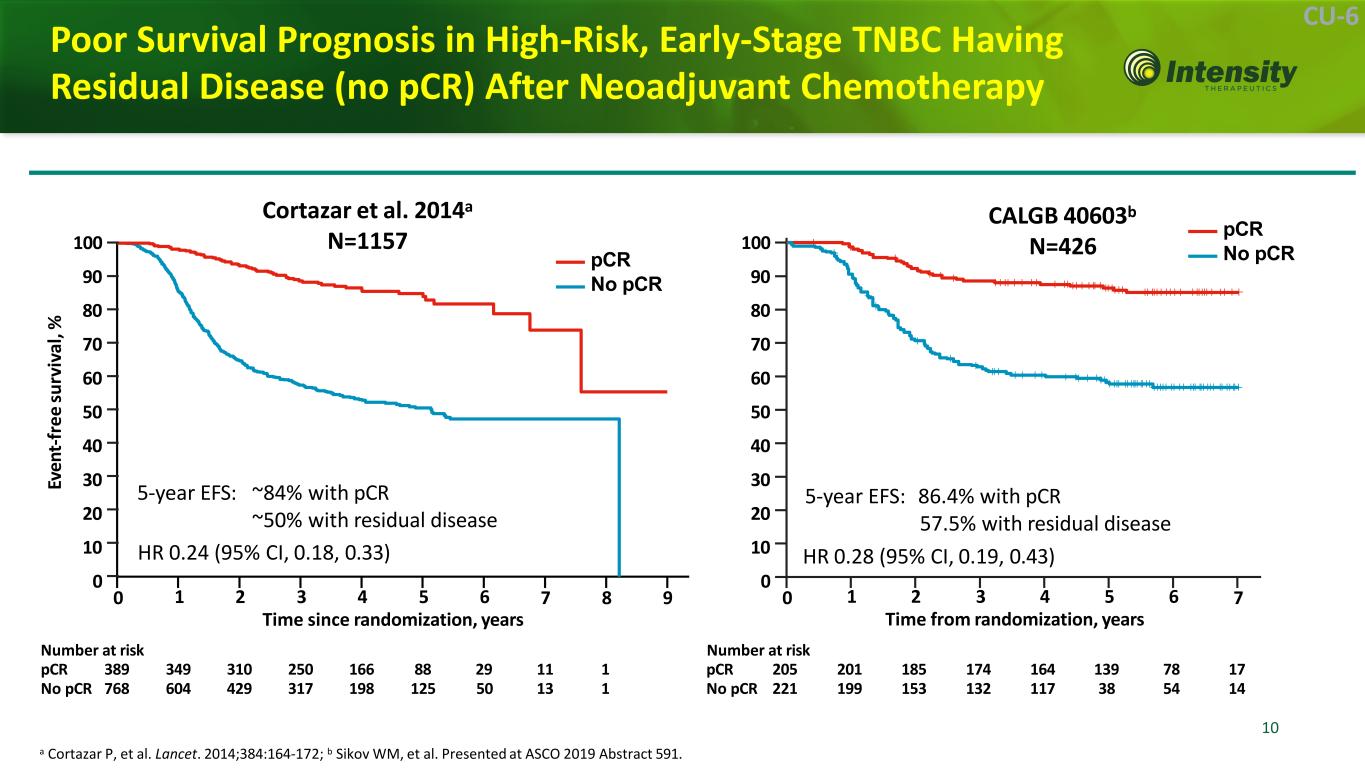
10 10 CU-6 Poor Survival Prognosis in High-Risk, Early-Stage TNBC Having Residual Disease (no pCR) After Neoadjuvant Chemotherapy a Cortazar P, et al. Lancet. 2014;384:164-172; b Sikov WM, et al. Presented at ASCO 2019 Abstract 591. CALGB 40603b N=426 Cortazar et al. 2014a N=1157 5-year EFS: ~84% with pCR ~50% with residual disease Number at risk Ev en t-f re e su rv iv al ,% 0 7 8 91 2 3 4 5 6 Time since randomization, years pCR No pCR 7 5-year EFS: 86.4% with pCR 57.5% with residual disease HR 0.28 (95% CI, 0.19, 0.43) 1 2 3 4 5 6 Time from randomization, years Number at risk pCR 389 349 310 250 166 88 29 11 1 pCR 205 201 185 174 164 139 78 17 No pCR 768 604 429 317 198 125 50 13 1 No pCR 221 199 153 132 117 38 54 14 0 100 90 80 70 60 50 40 30 20 10 0 HR 0.24 (95% CI, 0.18, 0.33) pCR No pCR100 90 80 70 60 50 40 30 20 10 0
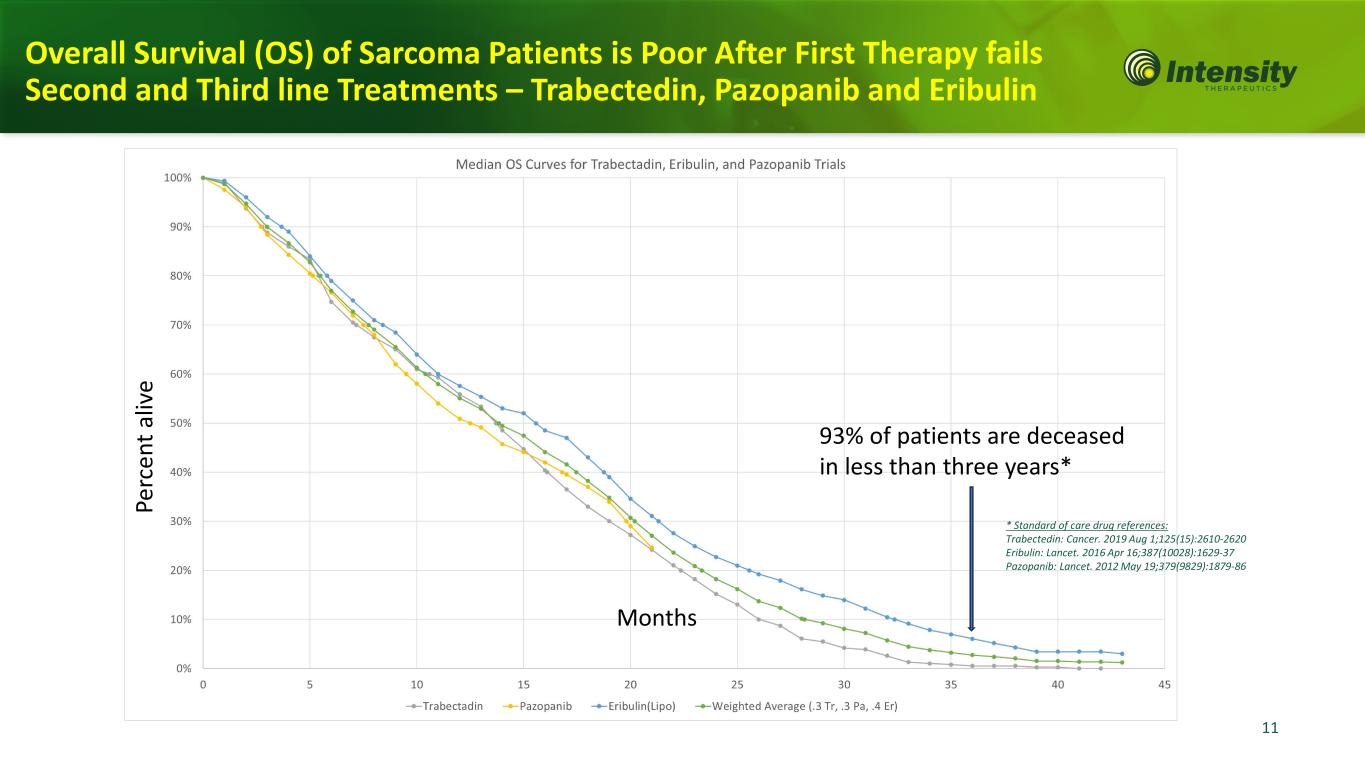
11 11 Overall Survival (OS) of Sarcoma Patients is Poor After First Therapy fails Second and Third line Treatments – Trabectedin, Pazopanib and Eribulin Pe rc en t a liv e Months 93% of patients are deceased in less than three years* * Standard of care drug references: Trabectedin: Cancer. 2019 Aug 1;125(15):2610-2620 Eribulin: Lancet. 2016 Apr 16;387(10028):1629-37 Pazopanib: Lancet. 2012 May 19;379(9829):1879-86
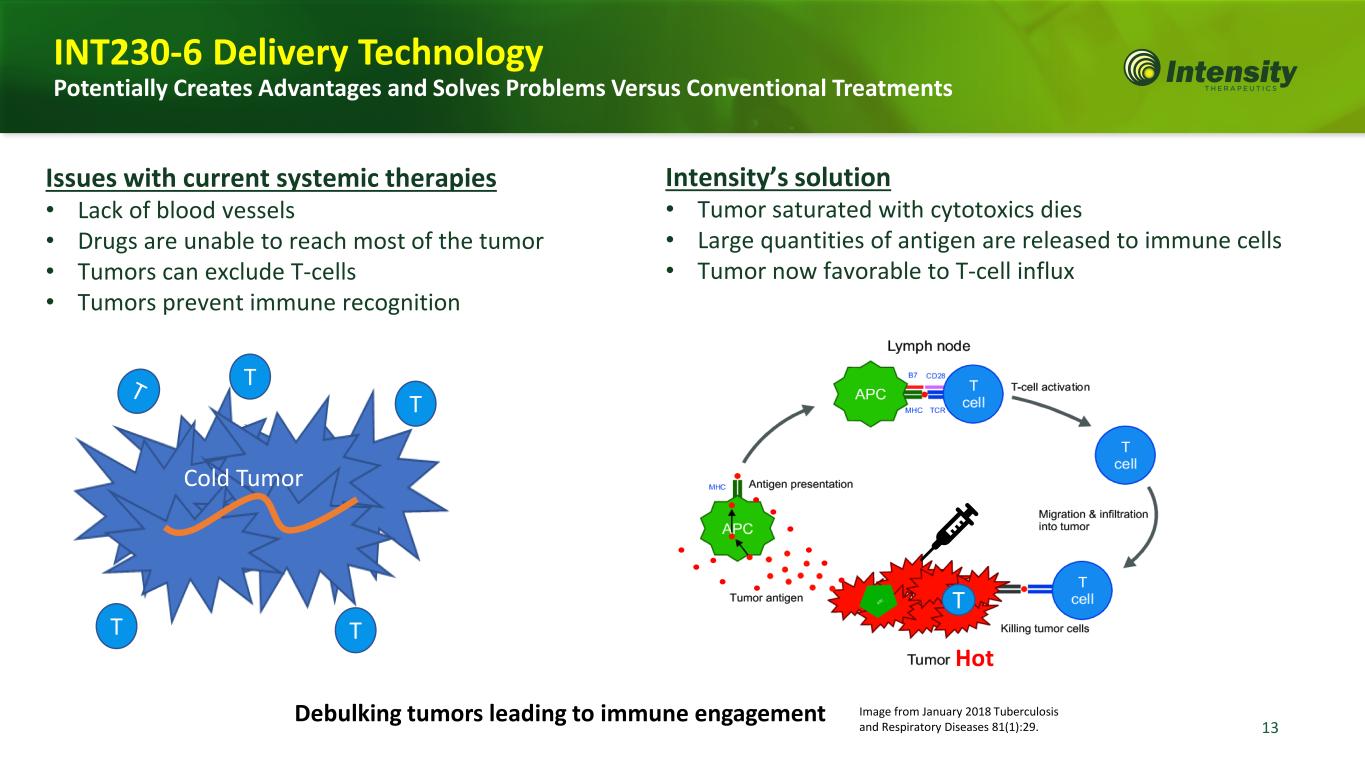
13 Intensity’s solution • Tumor saturated with cytotoxics dies • Large quantities of antigen are released to immune cells • Tumor now favorable to T-cell influx Issues with current systemic therapies • Lack of blood vessels • Drugs are unable to reach most of the tumor • Tumors can exclude T-cells • Tumors prevent immune recognition Hot T Image from January 2018 Tuberculosis and Respiratory Diseases 81(1):29. T T T T Cold Tumor INT230-6 Delivery Technology Potentially Creates Advantages and Solves Problems Versus Conventional Treatments Debulking tumors leading to immune engagement
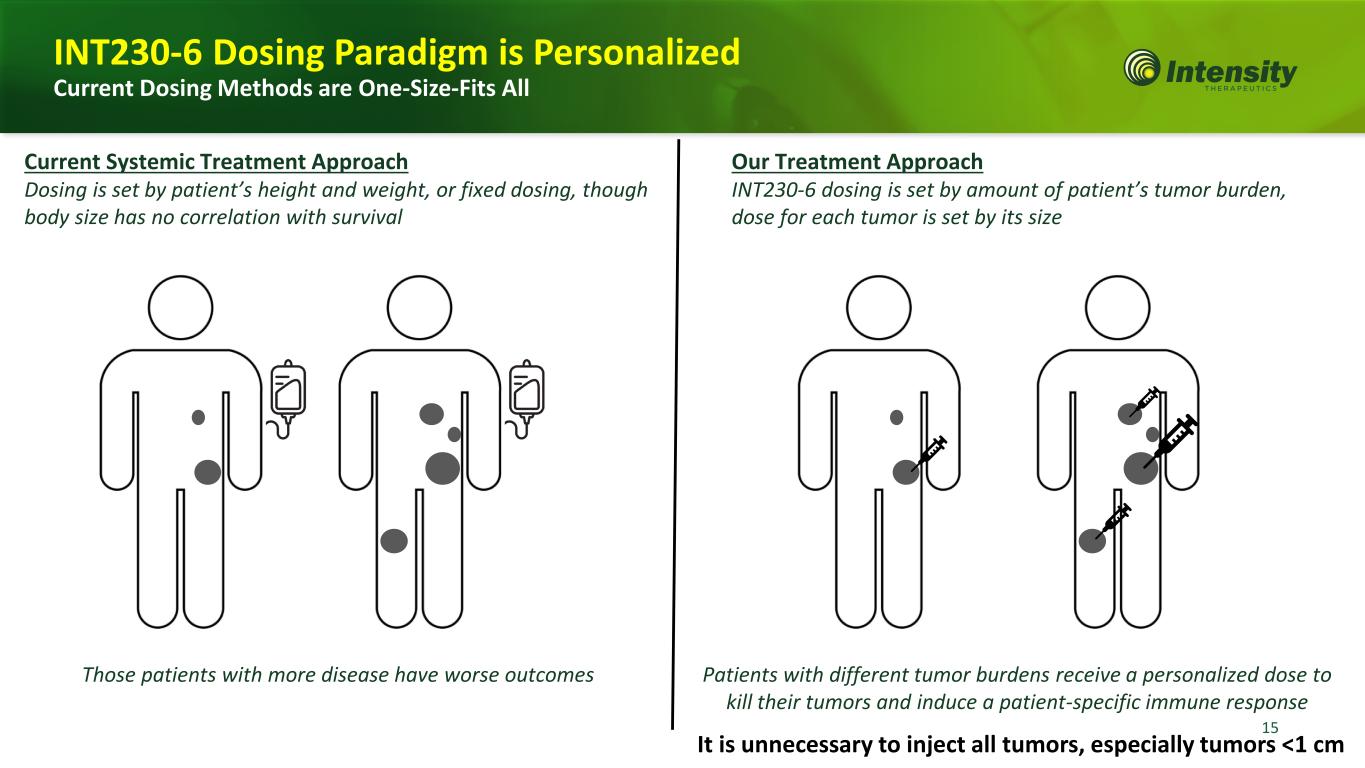
15 Current Systemic Treatment Approach Dosing is set by patient’s height and weight, or fixed dosing, though body size has no correlation with survival Our Treatment Approach INT230-6 dosing is set by amount of patient’s tumor burden, dose for each tumor is set by its size Those patients with more disease have worse outcomes Patients with different tumor burdens receive a personalized dose to kill their tumors and induce a patient-specific immune response INT230-6 Dosing Paradigm is Personalized Current Dosing Methods are One-Size-Fits All It is unnecessary to inject all tumors, especially tumors <1 cm
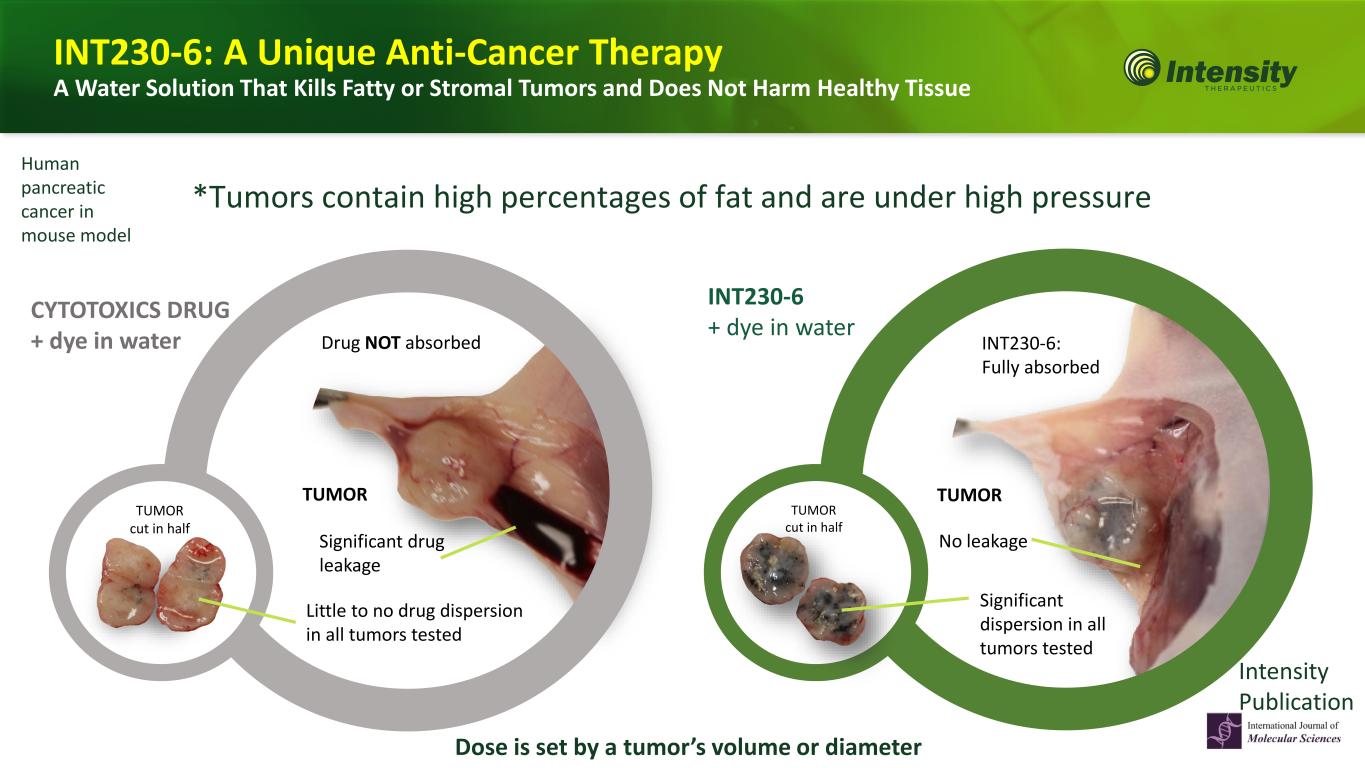
16 *Tumors contain high percentages of fat and are under high pressure CYTOTOXICS DRUG + dye in water Little to no drug dispersion in all tumors tested TUMOR cut in half Significant drug leakage Human pancreatic cancer in mouse model Drug NOT absorbed Human pancreatic cancer in mouse model TUMOR cut in half Significant dispersion in all tumors tested No leakage INT230-6: Fully absorbed INT230-6 + dye in water Dose is set by a tumor’s volume or diameter TUMOR TUMOR Intensity Publication INT230-6: A Unique Anti-Cancer Therapy A Water Solution That Kills Fatty or Stromal Tumors and Does Not Harm Healthy Tissue

17 INT230-6 for Presurgical Breast Cancer
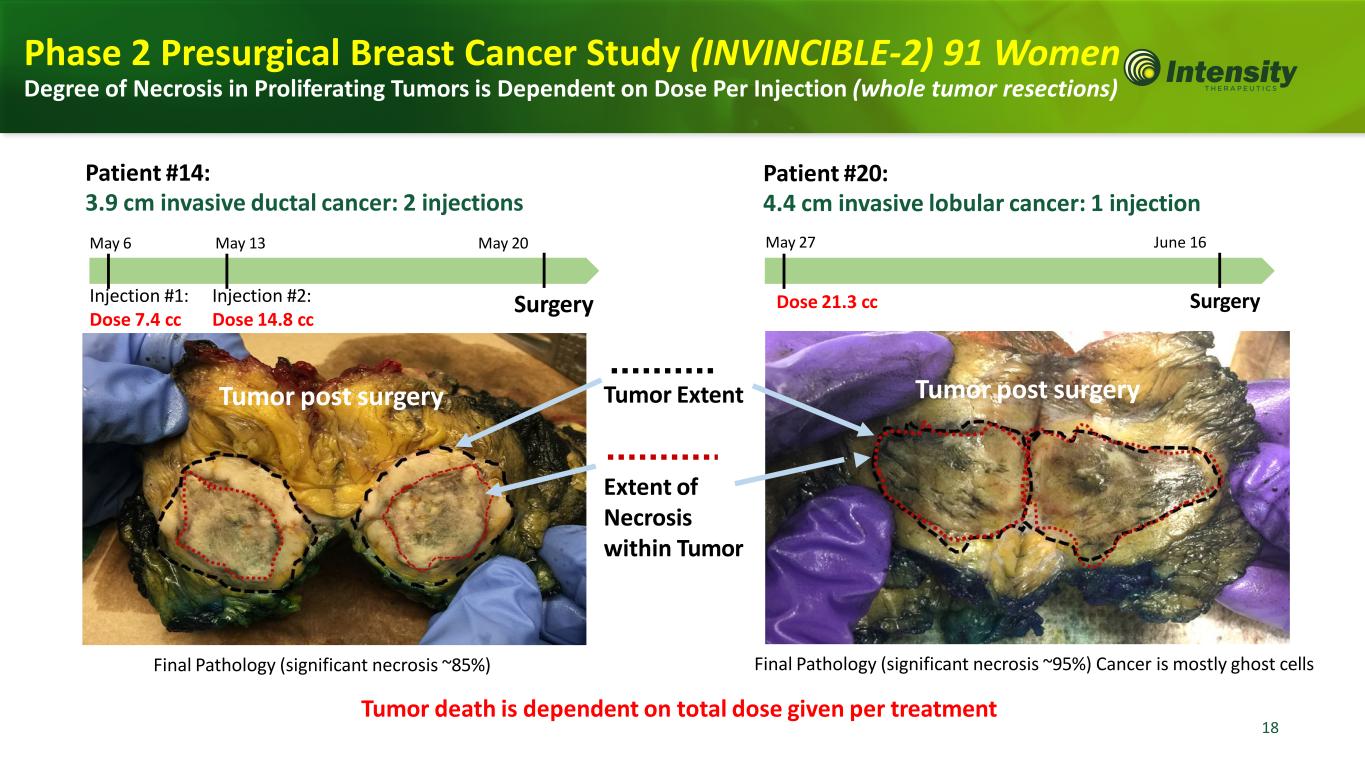
18 18 Tumor Extent Extent of Necrosis within Tumor Patient #14: 3.9 cm invasive ductal cancer: 2 injections May 6 May 13 May 20 Final Pathology (significant necrosis ~85%) Final Pathology (significant necrosis ~95%) Cancer is mostly ghost cells Injection #1: Dose 7.4 cc Injection #2: Dose 14.8 cc Surgery Patient #20: 4.4 cm invasive lobular cancer: 1 injection May 27 June 16 Dose 21.3 cc Surgery Tumor death is dependent on total dose given per treatment Tumor post surgery Tumor post surgery Phase 2 Presurgical Breast Cancer Study (INVINCIBLE-2) 91 Women Degree of Necrosis in Proliferating Tumors is Dependent on Dose Per Injection (whole tumor resections)
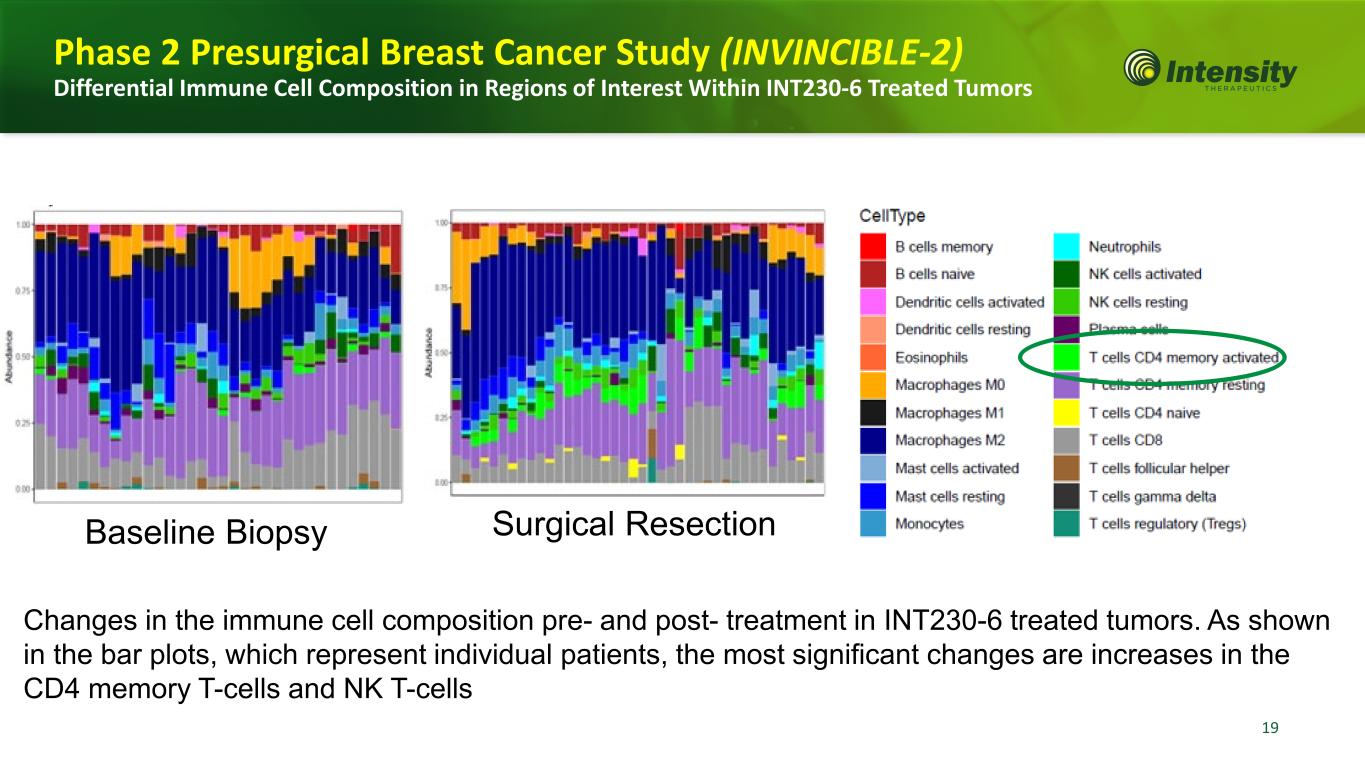
19 19 Changes in the immune cell composition pre- and post- treatment in INT230-6 treated tumors. As shown in the bar plots, which represent individual patients, the most significant changes are increases in the CD4 memory T-cells and NK T-cells Baseline Biopsy Surgical Resection Phase 2 Presurgical Breast Cancer Study (INVINCIBLE-2) Differential Immune Cell Composition in Regions of Interest Within INT230-6 Treated Tumors
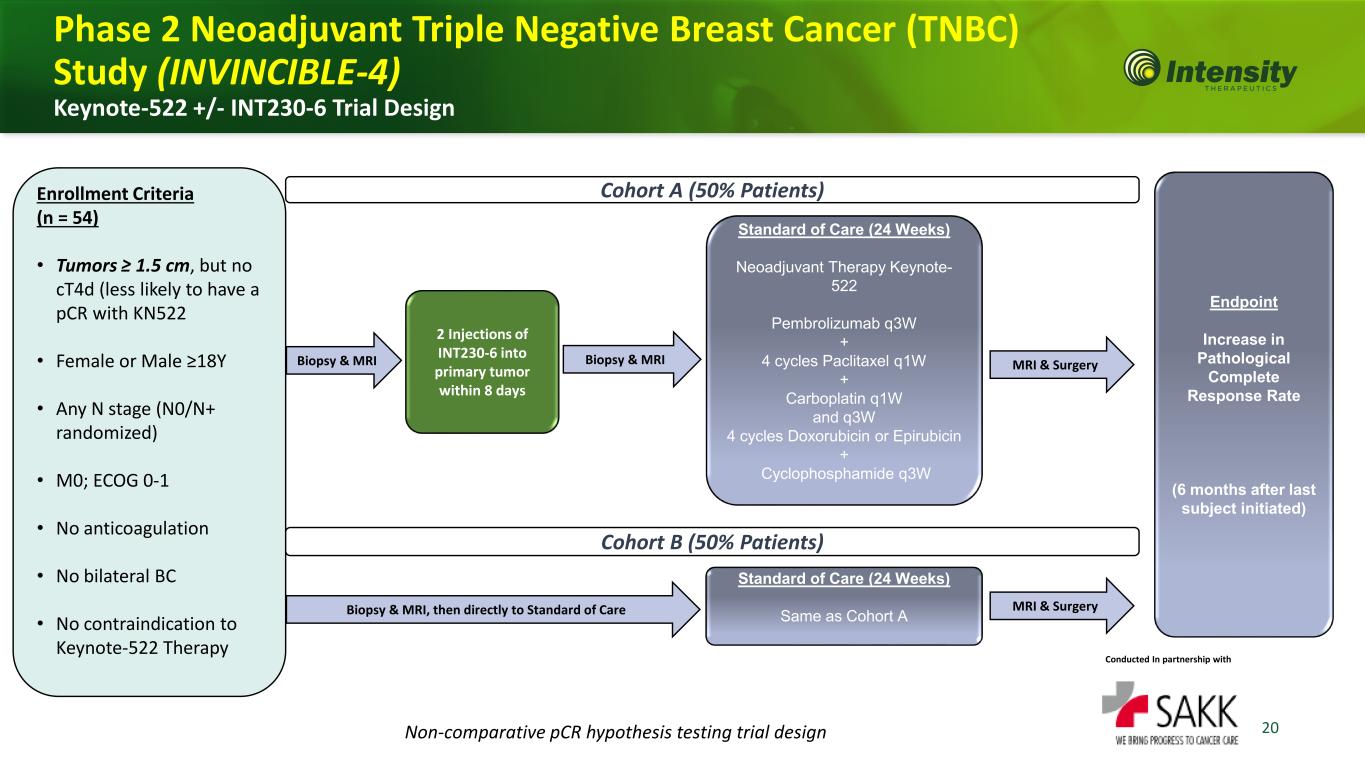
20 Enrollment Criteria (n = 54) • Tumors ≥ 1.5 cm, but no cT4d (less likely to have a pCR with KN522 • Female or Male ≥18Y • Any N stage (N0/N+ randomized) • M0; ECOG 0-1 • No anticoagulation • No bilateral BC • No contraindication to Keynote-522 Therapy Standard of Care (24 Weeks) Neoadjuvant Therapy Keynote- 522 Pembrolizumab q3W + 4 cycles Paclitaxel q1W + Carboplatin q1W and q3W 4 cycles Doxorubicin or Epirubicin + Cyclophosphamide q3W Cohort A (50% Patients) Phase 2 Neoadjuvant Triple Negative Breast Cancer (TNBC) Study (INVINCIBLE-4) Keynote-522 +/- INT230-6 Trial Design Biopsy & MRI 2 Injections of INT230-6 into primary tumor within 8 days Biopsy & MRI, then directly to Standard of Care Cohort B (50% Patients) Conducted In partnership with Standard of Care (24 Weeks) Same as Cohort A Endpoint Increase in Pathological Complete Response Rate (6 months after last subject initiated) MRI & Surgery MRI & Surgery Non-comparative pCR hypothesis testing trial design Biopsy & MRI
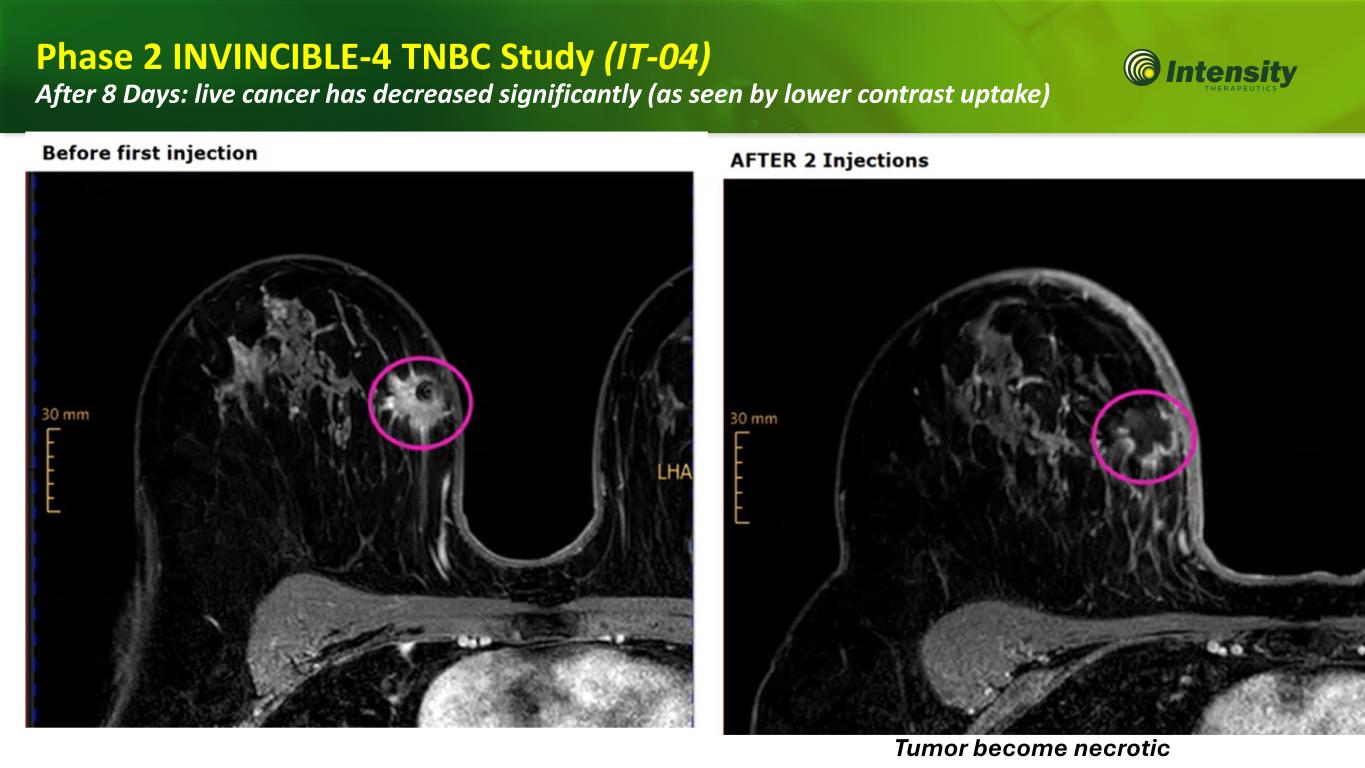
21 Phase 2 INVINCIBLE-4 TNBC Study (IT-04) After 8 Days: live cancer has decreased significantly (as seen by lower contrast uptake) Tumor become necrotic

23 INT230-6 in Metastatic Cancers
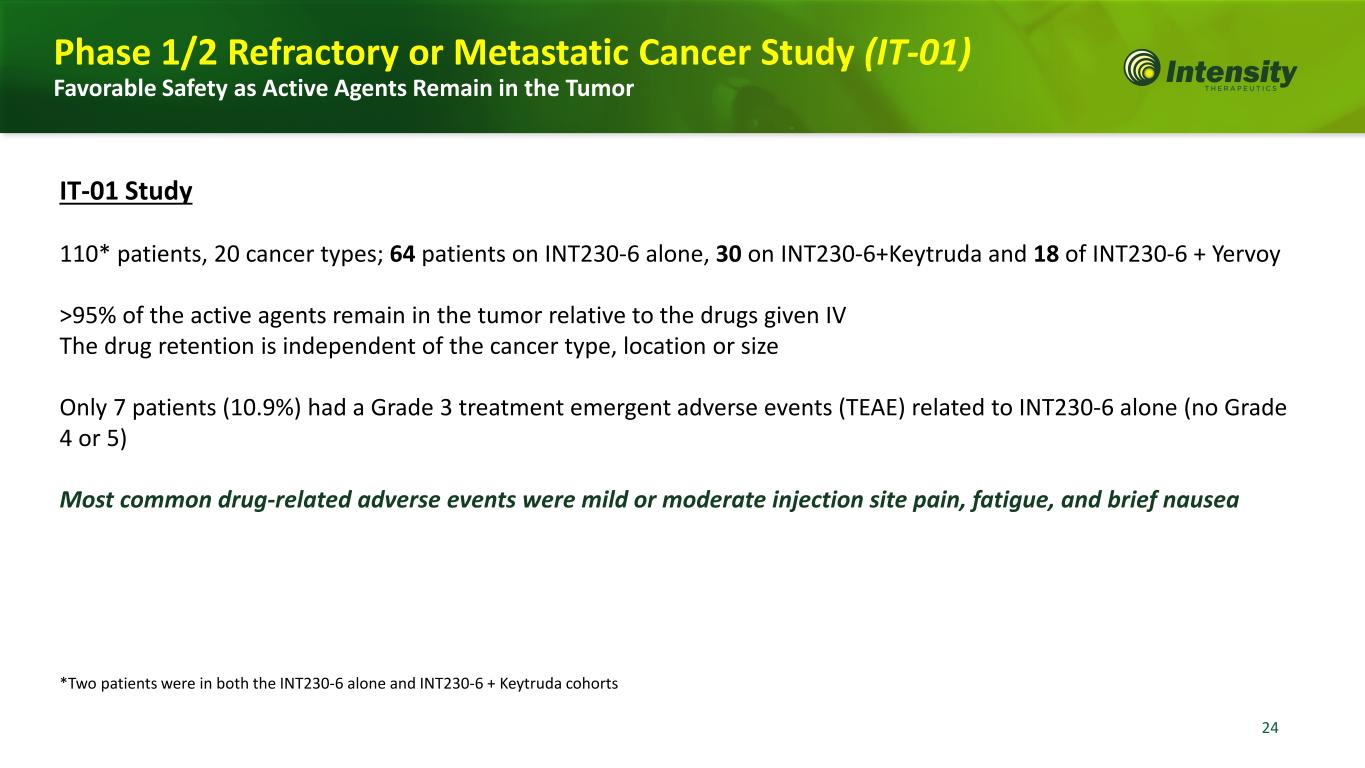
24 *Two patients were in both the INT230-6 alone and INT230-6 + Keytruda cohorts IT-01 Study 110* patients, 20 cancer types; 64 patients on INT230-6 alone, 30 on INT230-6+Keytruda and 18 of INT230-6 + Yervoy >95% of the active agents remain in the tumor relative to the drugs given IV The drug retention is independent of the cancer type, location or size Only 7 patients (10.9%) had a Grade 3 treatment emergent adverse events (TEAE) related to INT230-6 alone (no Grade 4 or 5) Most common drug-related adverse events were mild or moderate injection site pain, fatigue, and brief nausea Phase 1/2 Refractory or Metastatic Cancer Study (IT-01) Favorable Safety as Active Agents Remain in the Tumor
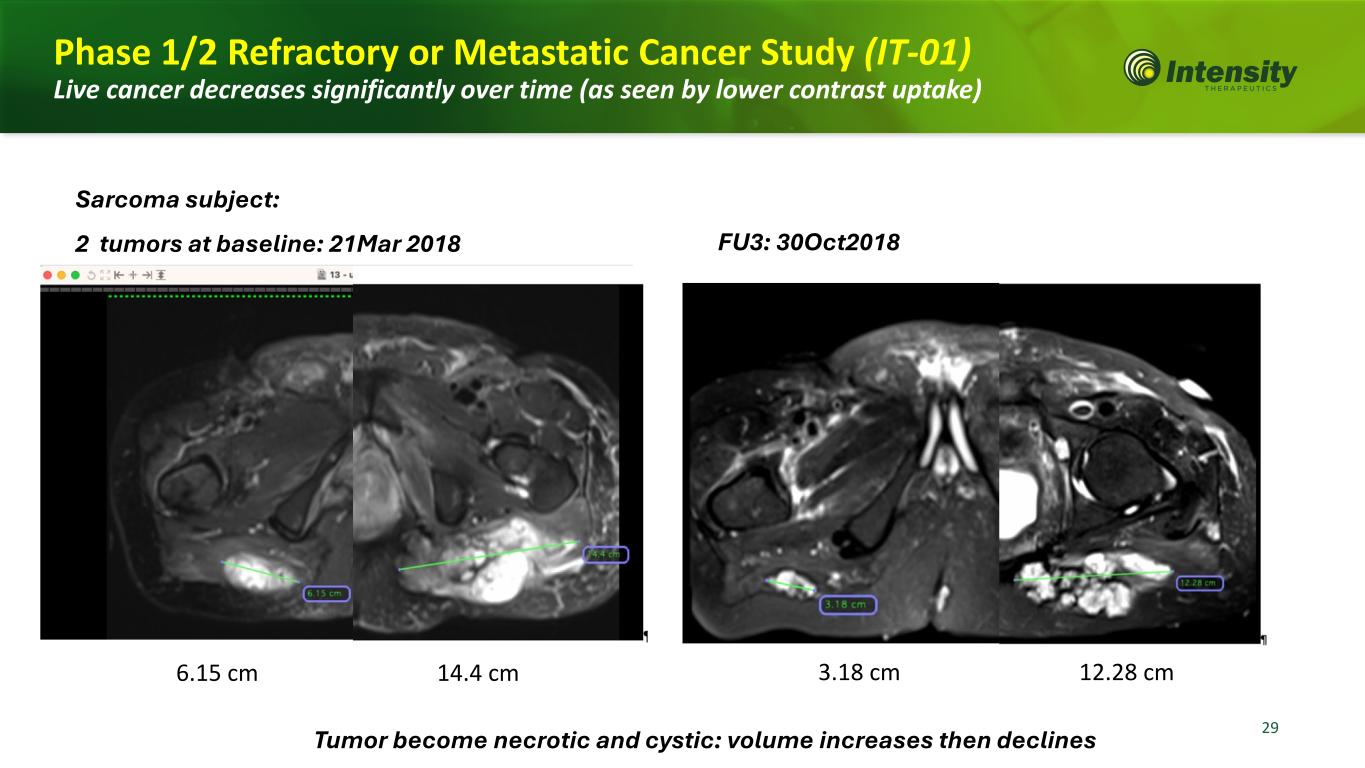
29 Phase 1/2 Refractory or Metastatic Cancer Study (IT-01) Live cancer decreases significantly over time (as seen by lower contrast uptake) 6.15 cm 14.4 cm 3.18 cm 12.28 cm Sarcoma subject: 2 tumors at baseline: 21Mar 2018 FU3: 30Oct2018 Tumor become necrotic and cystic: volume increases then declines
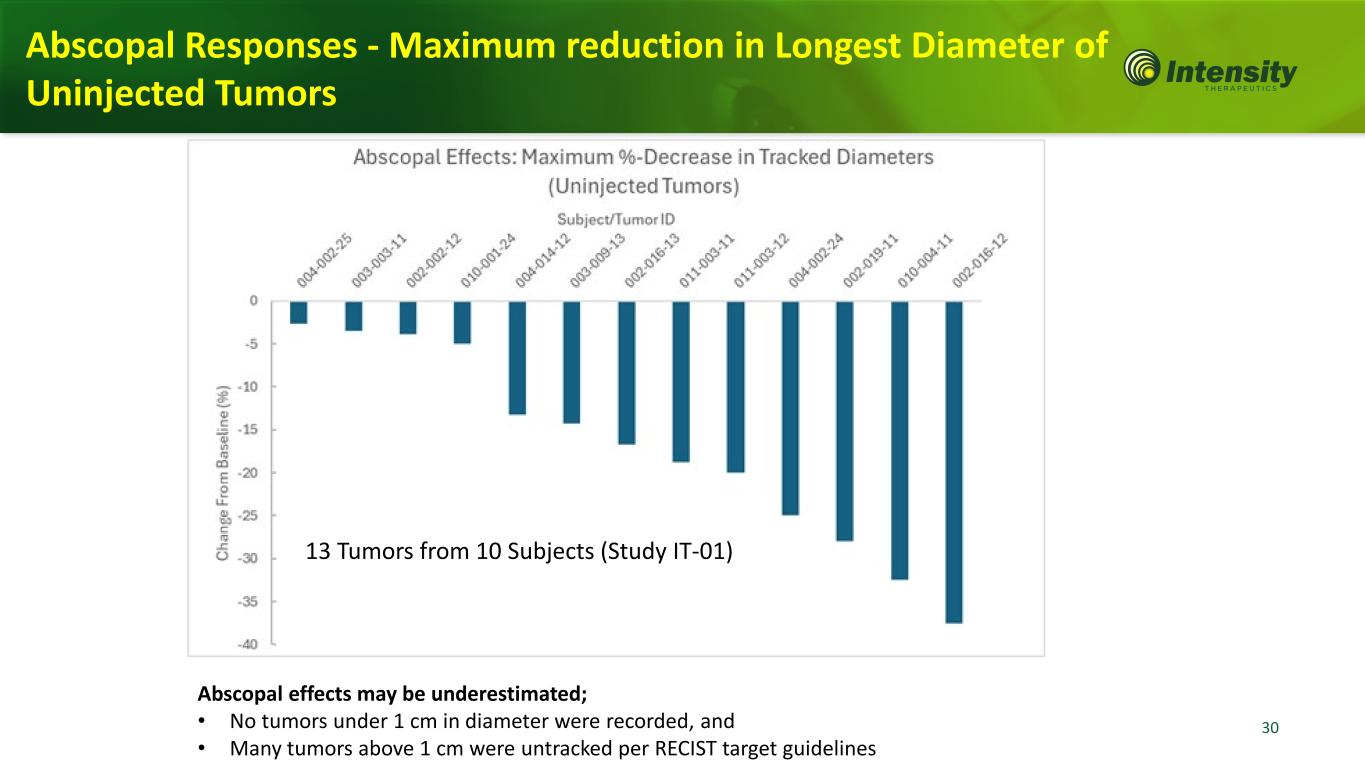
30 Abscopal effects may be underestimated; • No tumors under 1 cm in diameter were recorded, and • Many tumors above 1 cm were untracked per RECIST target guidelines Abscopal Responses - Maximum reduction in Longest Diameter of Uninjected Tumors 13 Tumors from 10 Subjects (Study IT-01)
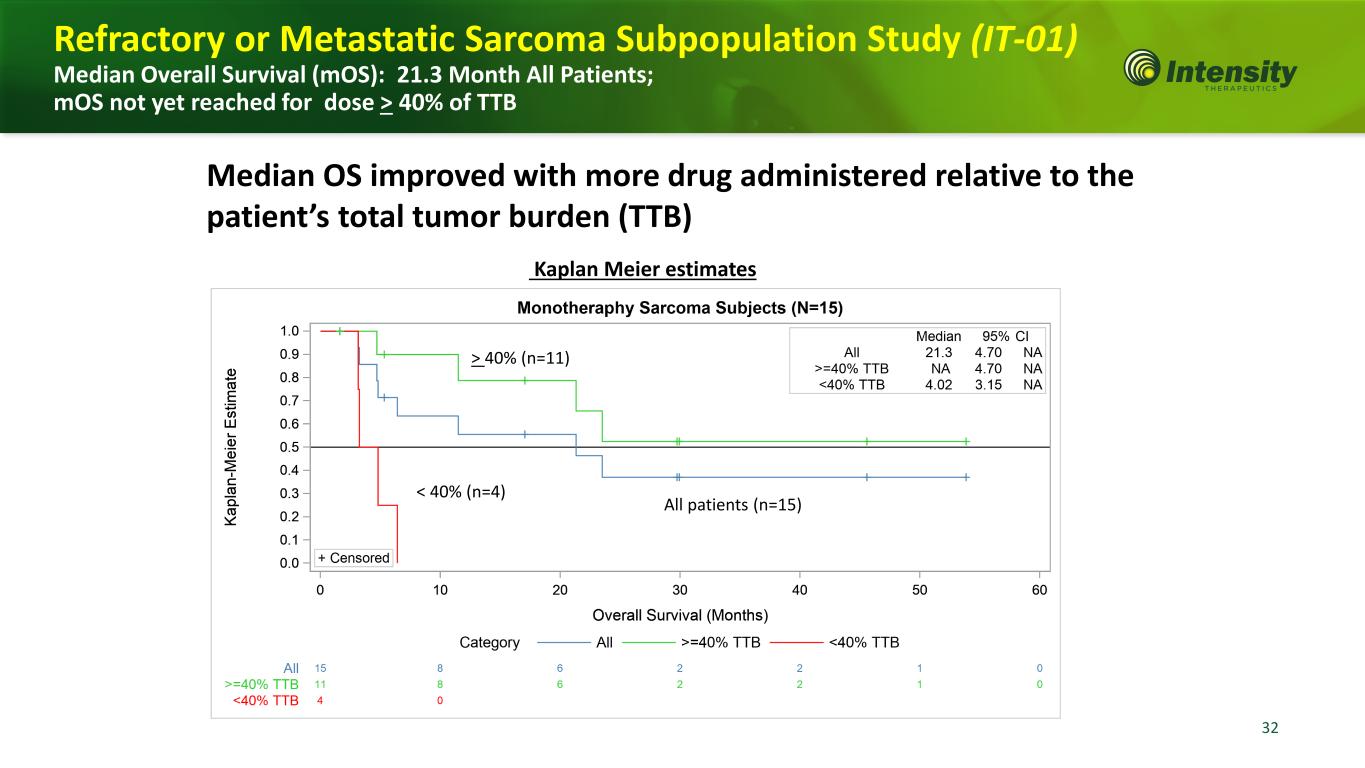
32 Kaplan Meier estimates Median OS improved with more drug administered relative to the patient’s total tumor burden (TTB) > 40% (n=11) All patients (n=15) < 40% (n=4) Refractory or Metastatic Sarcoma Subpopulation Study (IT-01) Median Overall Survival (mOS): 21.3 Month All Patients; mOS not yet reached for dose > 40% of TTB
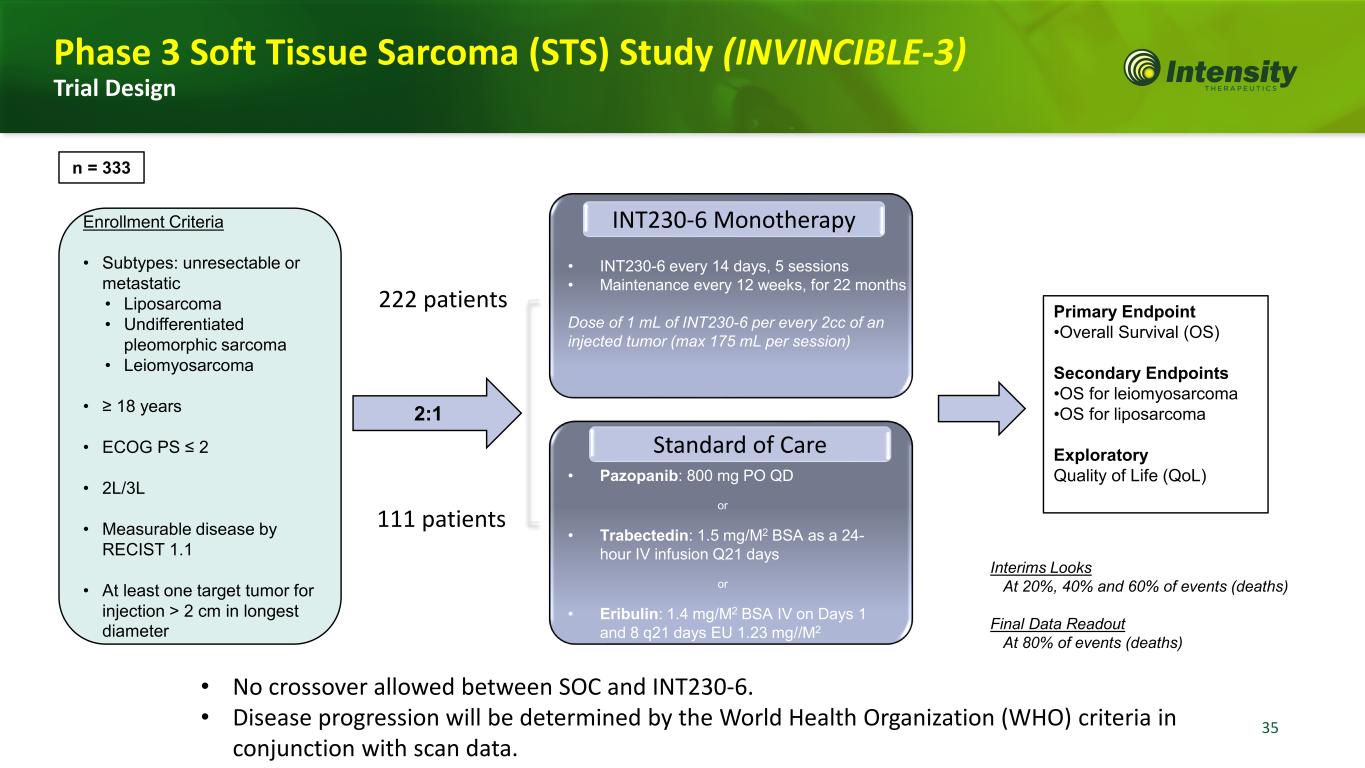
35 Interims Looks At 20%, 40% and 60% of events (deaths) Final Data Readout At 80% of events (deaths) Enrollment Criteria • Subtypes: unresectable or metastatic • Liposarcoma • Undifferentiated pleomorphic sarcoma • Leiomyosarcoma • ≥ 18 years • ECOG PS ≤ 2 • 2L/3L • Measurable disease by RECIST 1.1 • At least one target tumor for injection > 2 cm in longest diameter 2:1 Primary Endpoint •Overall Survival (OS) Secondary Endpoints •OS for leiomyosarcoma •OS for liposarcoma Exploratory Quality of Life (QoL) n = 333 • Pazopanib: 800 mg PO QD or • Trabectedin: 1.5 mg/M2 BSA as a 24- hour IV infusion Q21 days or • Eribulin: 1.4 mg/M2 BSA IV on Days 1 and 8 q21 days EU 1.23 mg//M2 Standard of Care INT230-6 Monotherapy • INT230-6 every 14 days, 5 sessions • Maintenance every 12 weeks, for 22 months Dose of 1 mL of INT230-6 per every 2cc of an injected tumor (max 175 mL per session) 222 patients 111 patients Phase 3 Soft Tissue Sarcoma (STS) Study (INVINCIBLE-3) Trial Design • No crossover allowed between SOC and INT230-6. • Disease progression will be determined by the World Health Organization (WHO) criteria in conjunction with scan data.
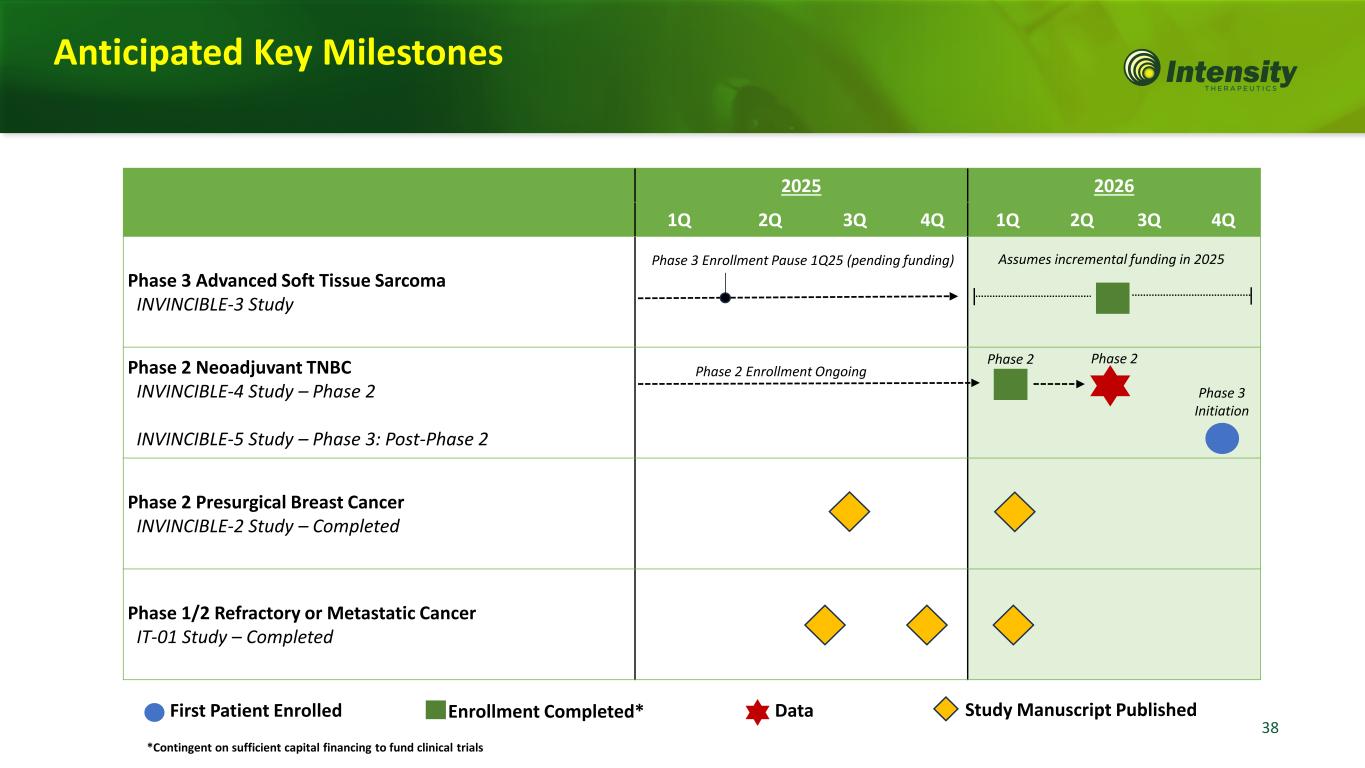
38 2025 2026 1Q 2Q 3Q 4Q 1Q 2Q 3Q 4Q Phase 3 Advanced Soft Tissue Sarcoma INVINCIBLE-3 Study Phase 2 Neoadjuvant TNBC INVINCIBLE-4 Study – Phase 2 INVINCIBLE-5 Study – Phase 3: Post-Phase 2 Phase 2 Presurgical Breast Cancer INVINCIBLE-2 Study – Completed Phase 1/2 Refractory or Metastatic Cancer IT-01 Study – Completed First Patient Enrolled Enrollment Completed* Data Phase 2 Phase 3 Initiation Phase 2 Study Manuscript Published Assumes incremental funding in 2025 Anticipated Key Milestones Phase 2 Enrollment Ongoing Phase 3 Enrollment Pause 1Q25 (pending funding) *Contingent on sufficient capital financing to fund clinical trials

39 Cash and Cash Equivalents(1) $2.6 million Debt(1) $ 0 Shares Outstanding(1): Common 15.1 million Options (weighted average exercise price: $6.14) 2.6 million Warrants (weighted average exercise price: $4.32) 2.0 million (1) As of December 31, 2024 Financial Highlights

40 • Solid cancers such as sarcoma and breast are challenging to treat due to the tumor’s physical properties • Intensity is a late-stage clinical biotech company developing a new delivery technology to overcome the barriers to cancer cell death • Intensity’s product candidate, INT230-6, can be used in the metastatic and presurgical (neoadjuvant) settings • Intensity is capital-efficient and focused on execution and achieving milestone • Sarcoma and breast cancer represent significant opportunities for revenue Summary

42 Lewis H. Bender, MIT ChE, MS, MA, MBA • Drug delivery expertise Preclinical through Phase 3 • Public biotech company CEO experience • Public CFO, 30 years, • Extensive M&A and financing transactions John Wesolowski, MBA, CPA Lewis H. Bender CEO Intensity Daniel Donovan CEO Rare Life Emer Leahy, Ph.D. CEO Psychogenics Mark A. Goldberg, MD Former President & COO of PAREXEL Thomas Dubin Former Chief Counsel Alexion Founder, CEO Principal Accounting Officer and Controller Chief Financial Officer Barbara Mohl VP, Human Resources Vice President, Clinical Operations Kimberly Guedes, RN, MBA Joseph Bernadino, Josh Rodriques Manufacturing API and Drug Product Rita Cooney Ph.D. Analytical Chemistry CEO, CTO, VP, BD & Manufacturing CEO Manufacturing Joseph Talamo BOARD OF DIRECTORSKEY MANAGEMENT Ian B. Walters, MD Chief Medical Officer • 25 years experience • Global Phase 3 Experience Centrexion James Ahlers Corporate Strategy Management Team Extensive Oncology, Drug Development, and Public Company Experience Doranne Frano VP, Regulatory & Quality

43 INTENSITY THERAPEUTICS A NEW WEAPON IN THE WAR ON CANCER Investor Contact: Justin Kulik CORE IR IntensityIR@coreIR.com Thank you!